Method for producing fertilizer monopotassium phosphate by phosphoric acid by semi-hydrated process and wet process
A technology of potassium dihydrogen phosphate and wet-process phosphoric acid, which is applied in the field of phosphorus chemical industry, can solve the problems of serious equipment corrosion, high production cost, short supply of potassium dihydrogen phosphate, etc., and achieves the effect of reducing water consumption and improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
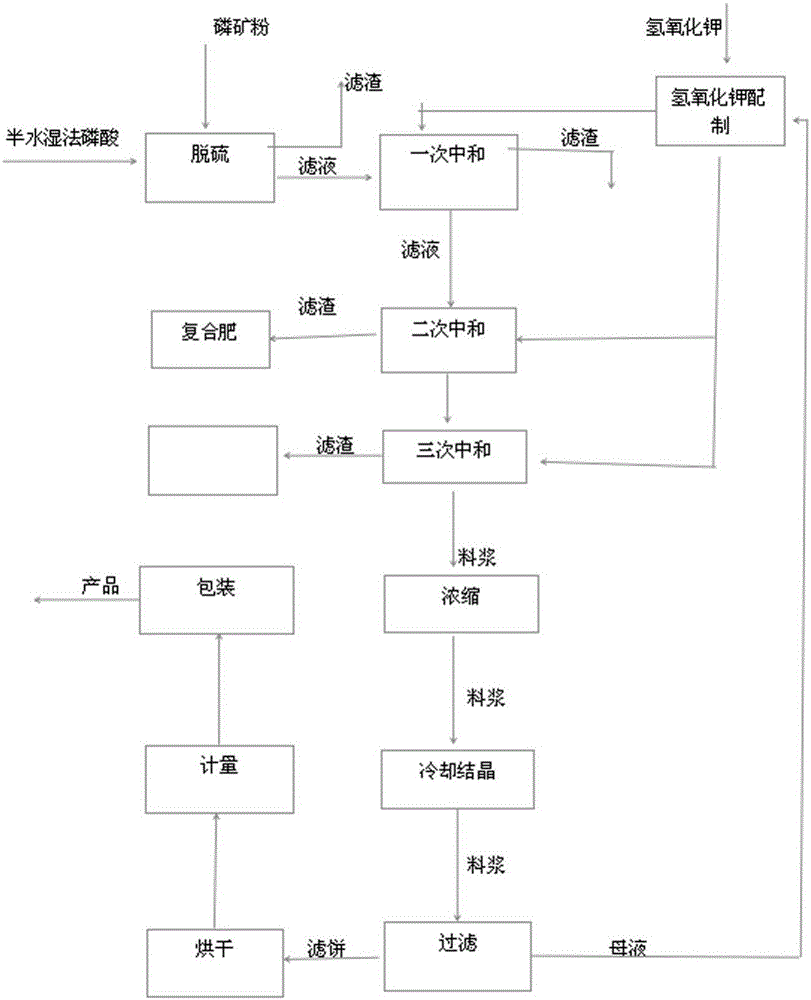
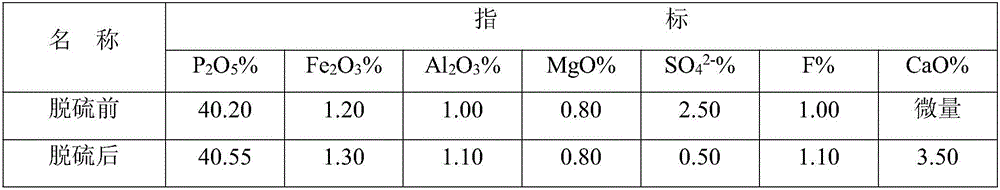
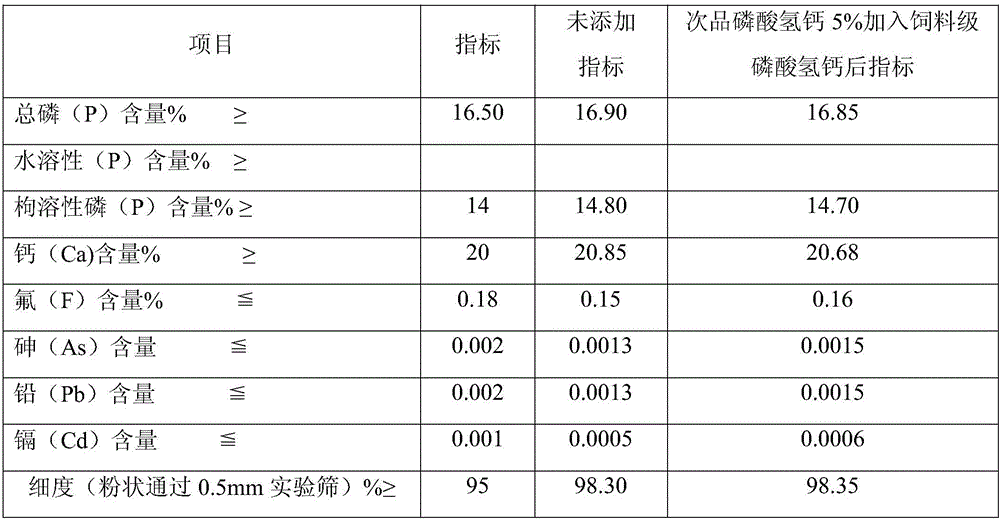
[0022] Add semi-aqueous wet-process phosphoric acid to phosphate rock powder for desulfurization, the addition ratio is 1:2 according to the molar ratio of sulfur and calcium oxide, and the reaction time is 1.5 hours. The reaction temperature is 50°C. The slurry after desulfurization is filtered, and the filter residue is sent to the phosphoric acid system. Therefore, the filtrate enters the primary neutralization tank, and the concentration is 15% potassium hydroxide is added for neutralization, and the pH value of the primary neutralization slurry is adjusted to 2.2. The reaction time 1.5 hours, the reaction temperature is 60°C, the primary neutralization slurry is filtered, the filter residue is added to the nitrogen, phosphorus and potassium compound fertilizer, the filtrate enters the secondary neutralization tank, and the concentration is 15% potassium hydroxide is added for secondary neutralization, and the secondary neutralization is adjusted. The pH value of the slurry...
Embodiment 2
[0026] Add semi-aqueous wet-process phosphoric acid to phosphate rock powder for desulfurization, the addition ratio is 1:3 according to the molar ratio of sulfur to calcium oxide, and the reaction time is 1.5 hours. The reaction temperature is 50°C. The slurry after desulfurization is filtered, and the filter residue is sent to the phosphoric acid system. Therefore, the filtrate enters the primary neutralization tank, and the concentration is 15% potassium hydroxide is added for neutralization, and the pH value of the primary neutralization slurry is adjusted to 2.2. The reaction time 1.5 hours, the reaction temperature is 30°C, the primary neutralization slurry is filtered, the filter residue is added to the nitrogen, phosphorus and potassium compound fertilizer, the filtrate enters the secondary neutralization tank, and the concentration is 15% potassium hydroxide is added for secondary neutralization, and the secondary neutralization is adjusted. The pH value of the mixed s...
Embodiment 3
[0029]
[0030] Add semi-aqueous wet-process phosphoric acid to phosphate rock powder for desulfurization, the addition ratio is 1:2.5 according to the molar ratio of sulfur to calcium oxide, and the reaction time is 1.5 hours. The reaction temperature is 50°C. The slurry after desulfurization is filtered, and the filter residue is sent to the phosphoric acid system. Therefore, the filtrate enters the primary neutralization tank, and the concentration is 15% potassium hydroxide is added for neutralization, and the pH value of the primary neutralization slurry is adjusted to 2.2. The reaction time 1.5 hours, the reaction temperature is 30°C, the primary neutralization slurry is filtered, the filter residue is added to the nitrogen, phosphorus and potassium compound fertilizer, the filtrate enters the secondary neutralization tank, and the concentration is 15% potassium hydroxide is added for secondary neutralization, and the secondary neutralization is adjusted. The pH value ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com