Method for preparing beta-tricalcium phosphate crystal material under low temperature condition
A technology for phase tricalcium phosphate and crystal materials, which is applied in the field of preparation of β-phase tricalcium phosphate crystal materials under low temperature conditions, can solve the problems of difficulty in synthesizing small-scale granular materials and high energy consumption, and achieve low reaction temperature and low energy consumption. The effect of less and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Solution preparation: weigh 4.44 grams of anhydrous CaCl 2 Dissolve in 400 ml of deionized water, stir well to obtain CaCl with a concentration of 0.1 mol / L 2 Solution, mix 4.06 grams of MgCl in the ratio of 1:2 according to the molar ratio of magnesium to calcium 2 ·6H 2 O powder, stir well to make MgCl 2 Completely dissolved to give CaCl 2 and MgCl 2 mixture. Weigh 4.24 g of anhydrous Na 2 CO 3 Dissolve in 400ml deionized water to prepare Na with a concentration of 0.1mol / L 2 CO 3 solution.
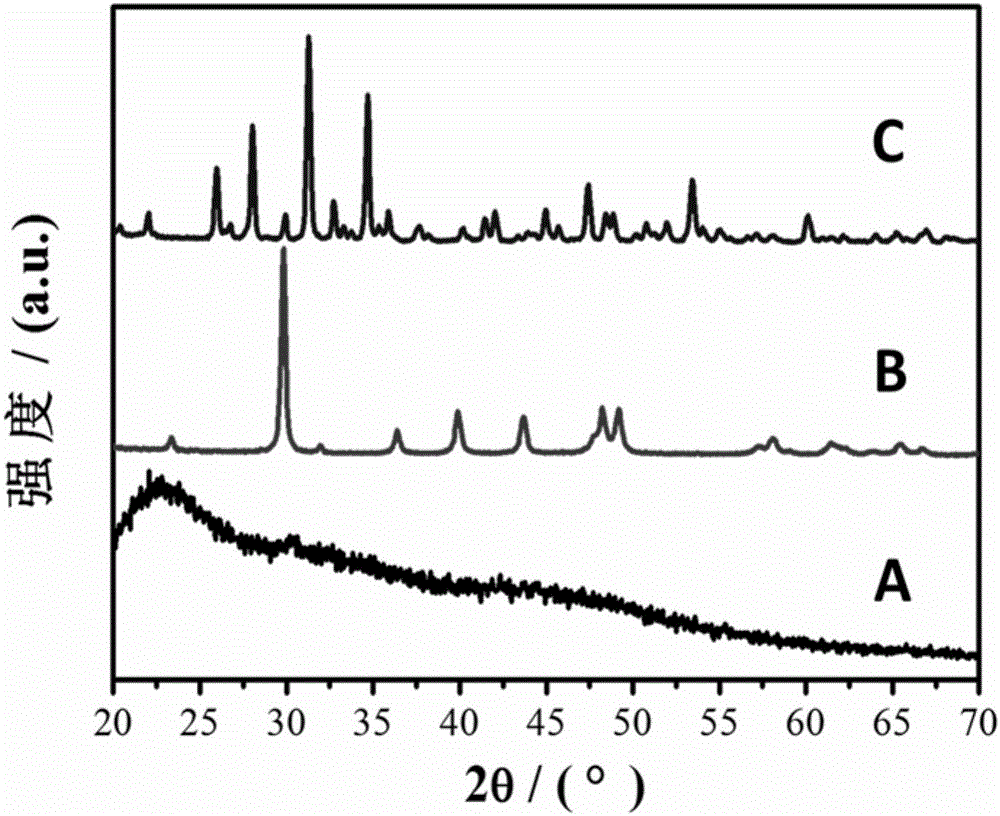
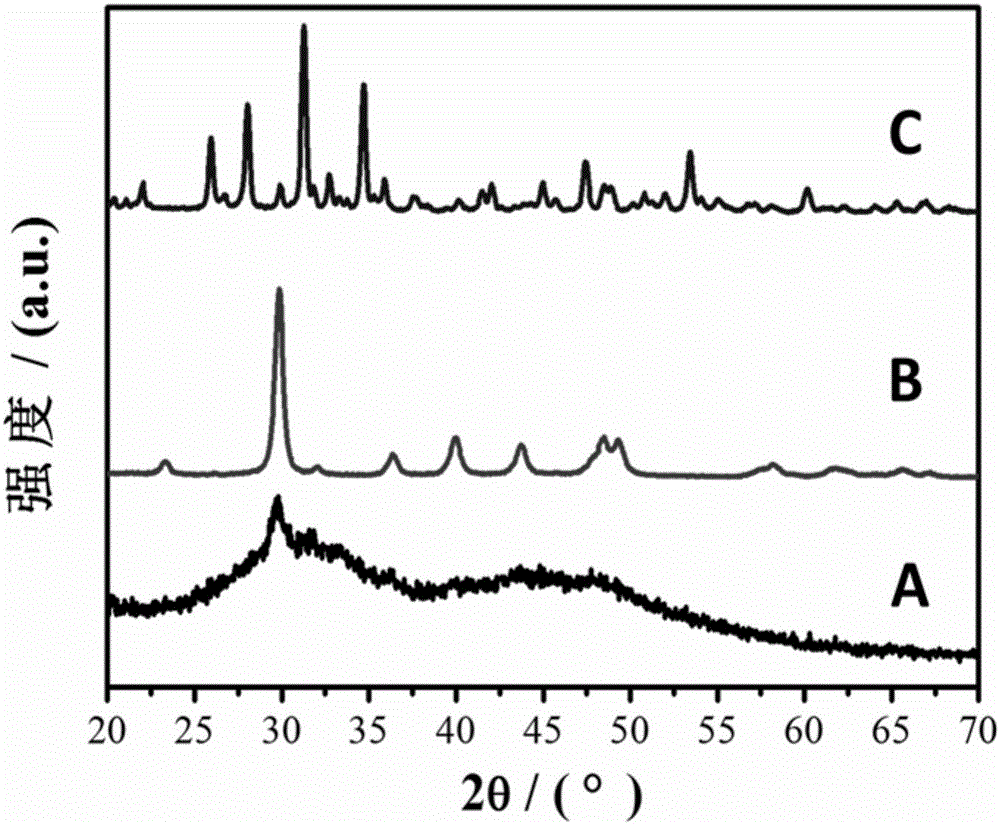
[0034] Rapid precipitation reaction: the prepared 400 ml CaCl 2 and MgCl 2 Mix the solution with 400 mL of Na 2 CO 3 The solutions were mixed, magnetically stirred (700rpm), and immediately poured into a Buchner funnel for suction filtration under reduced pressure, followed by washing with deionized water for 3 times and absolute ethanol for 1 time. Obtain Mg-containing amorphous calcium carbonate ( figure 1 (A)).
[0035] Crystallization of amorphous calcium carb...
Embodiment 2
[0039] Solution preparation: weigh 4.44 grams of anhydrous CaCl 2 Dissolve in 400 ml of deionized water, stir well to obtain CaCl with a concentration of 0.1 mol / L 2 solution, and then mixed with 8.12 grams of MgCl according to the ratio of magnesium to calcium molar ratio of 1:1 2 ·6H 2 O powder, stir well to make MgCl 2 Completely dissolved to give CaCl 2 and MgCl 2 mixture. And weigh 4.24 grams of anhydrous Na 2 CO 3 Dissolve in 400ml deionized water to prepare Na with a concentration of 0.1mol / L 2 CO 3 solution.
[0040] Rapid precipitation reaction: the prepared 400 ml CaCl 2 and MgCl 2 Mix the solution with 400 mL of Na 2 CO 3 The solutions were mixed, stirred evenly by magnetic force (700rpm), immediately poured into a Buchner funnel for vacuum filtration, and then washed with deionized water for 3 times and absolute ethanol for 1 time. Obtain Mg-containing amorphous calcium carbonate ( figure 2 (A); Figure 5 (A, B)).
[0041] Amorphous calcium carbon...
Embodiment 3
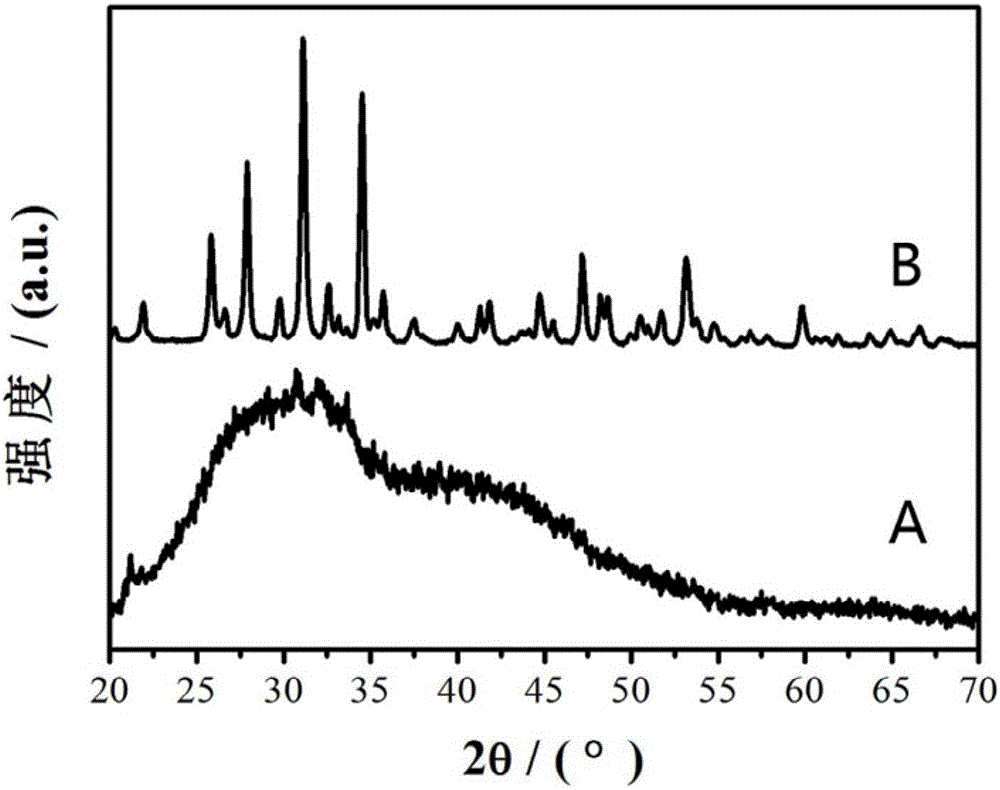
[0046] Solution preparation: Weigh 4.11 grams of Ca(NO 3 ) 2 4H 2 O was dissolved in 20 ml of deionized water, and the Ca(NO 3 ) 2 solution, then according to the ratio of calcium to magnesium molar ratio of 9:1 mixed with 0.49g gram of Mg (NO 3 ) 2 ·6H 2 O powder, stir well to make Mg(NO 3 ) 2 Completely dissolved, Ca(NO 3 ) 2 and Mg(NO 3 ) 2 The solution was mixed and its pH was adjusted to 10 with 25 wt.% ammonia solution. and weighed 1.54 g (NH 4 ) 2 HPO 4 Dissolve in 20 ml of deionized water to prepare a concentration of 0.64mol / L (NH 4 ) 2 HPO 4 Solution, its pH value was adjusted to 10 with 25wt.% ammonia solution. 20 ml of the prepared Ca(NO 3 ) 2 and Mg(NO 3 ) 2 Mix the solution with 20 ml (NH 4 ) 2 HPO 4 Solution mixing, magnetic stirring (300rpm) is even, obtains the amorphous apatite ( image 3 (A)) Emulsion.
[0047] Hydrothermal synthesis reaction: place the amorphous apatite emulsion containing 10% Mg in a hydrothermal reaction kettle,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com