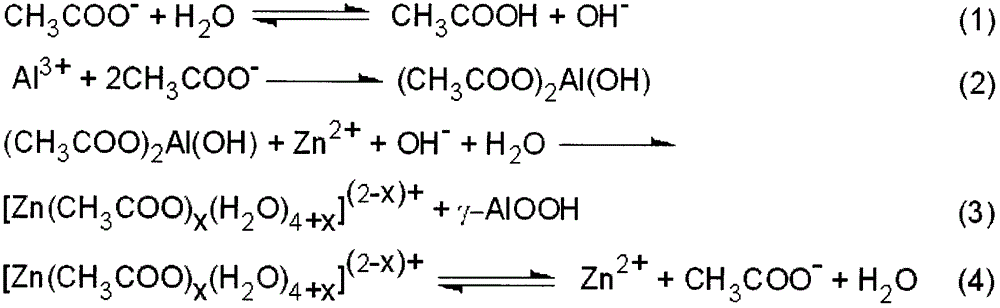Preparation method of gamma-AlOOH and gamma-Al2O3 nanotube and nanostructure
A nanostructured and mild technology, applied in alumina/hydroxide preparation, nanotechnology for materials and surface science, nanotechnology, etc., can solve the complex product purification process, product microstructure damage, surface activity reduction, etc. problem, to achieve the effect of realizing product morphology, reducing formation temperature, and mild preparation reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The preparation steps are as described in the summary of the invention, 5mol 1-butyl-2,3-dimethylimidazolium chloride ([Bdmim]Cl) ionic liquid, the reaction temperature is 130°C, and the reaction time is 10h. After the reaction, it was naturally cooled to room temperature. The product was filtered, washed with deionized water and absolute ethanol, and dried in a vacuum oven at 80° C. for 3 hours to obtain γ-alumina oxyhydroxide nanomaterials. The γ-alumina oxyhydroxide precursor was placed in a ceramic crucible, and calcined in a muffle furnace at 600° C. for 2 hours to obtain γ-alumina nanomaterials.
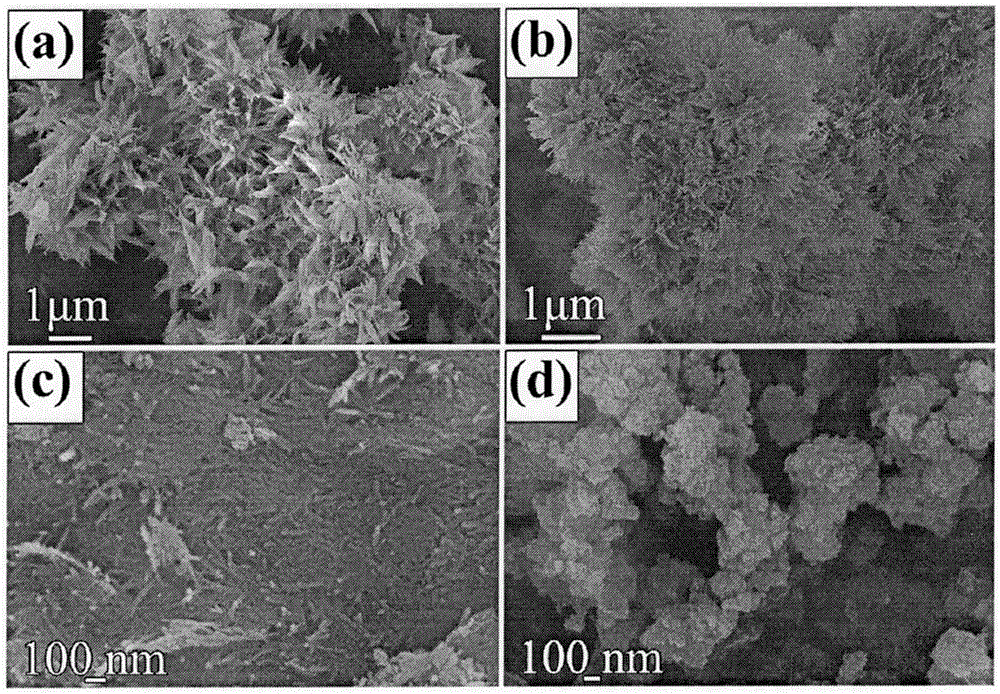
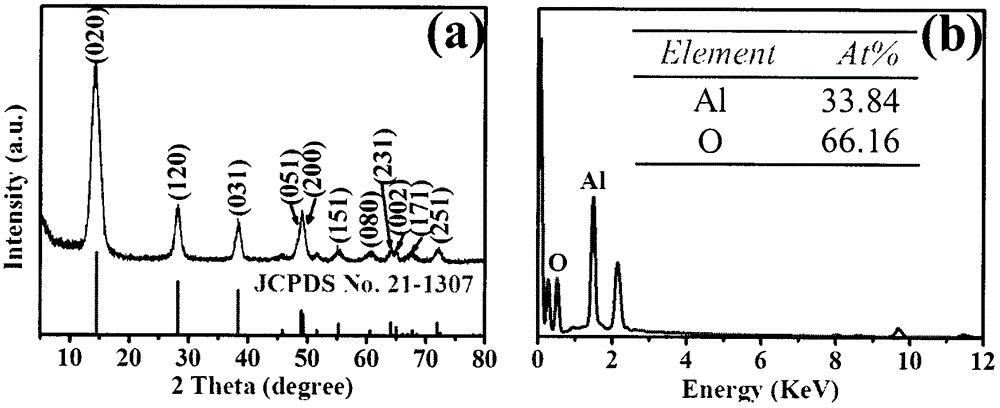
[0035] The obtained γ-alumina oxyhydroxide and γ-alumina are respectively orthogonal γ-alumina oxyhydroxide (JCPDS Card 21-1307) and cubic γ-alumina (JCPDS Card 50-0741). The XRD spectrum and elemental analysis are shown in the attached figure 2 And attached image 3 Shown, and energy spectrum analysis could not detect zinc. Scanning and transmission electron microsc...
Embodiment 2
[0037] Reaction condition is the same as embodiment 1, only replaces zinc nitrate with the magnesium nitrate of equimolar. XRD and electron spectrum test results of the synthesized product, as attached Figure 7 shown. The XRD pattern of the prepared product is similar to the product of Example 1, which can be attributed to the orthogonal structure of γ-AlOOH (JCPDS Card21-1307), and the results of elemental analysis with electron spectroscopy show that no magnesium element product can be detected in the product. The scanning electron microscope analysis result of product shows, product appearance is also similar to embodiment 1 product (attachment Figure 8 ).
Embodiment 3
[0039] The reaction conditions are the same as in Example 1, only the reaction temperature is increased to 150°C. After the reaction, it was naturally cooled to room temperature. The product was filtered, washed with deionized water and absolute ethanol, and dried in a vacuum oven at 80° C. for 3 hours to obtain γ-alumina oxyhydroxide nanomaterials. The γ-alumina oxyhydroxide was placed in a ceramic crucible, and calcined in a muffle furnace at 600° C. for 2 hours to obtain γ-alumina nanomaterials.
[0040] The physical phase of gained gamma-alumina oxyhydroxide and gamma-alumina is identical with embodiment 1 (attachment Figure 9 And attached Figure 11 ), but the shape is bamboo leaf-like nanoflower structure (attached Figure 10 And attached Figure 11 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com