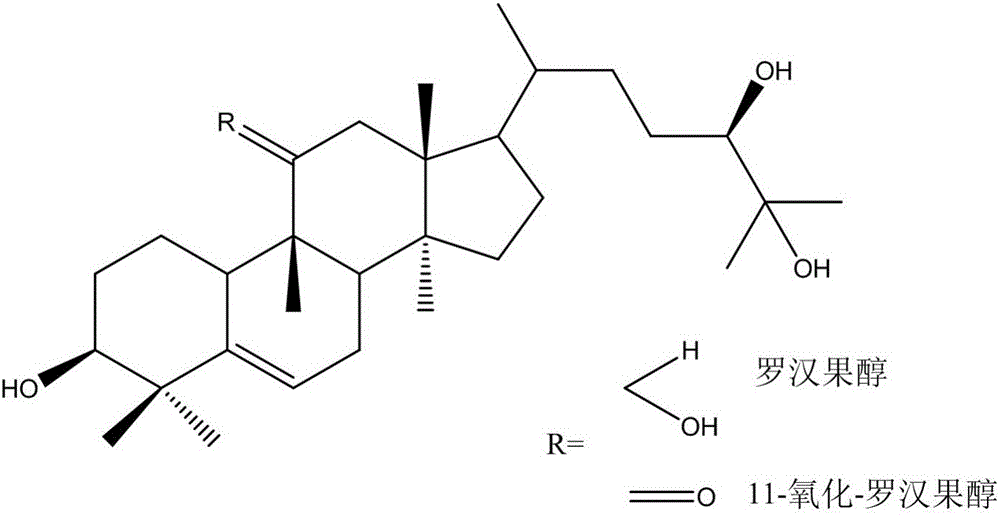
Method for preparing mogrol and 11-O-mogrol by combining enzyme hydrolysis and oxidative cracking
A technology of grosvenorol and oxidative cracking, applied in the direction of fermentation, etc., can solve the problems of not suitable for the preparation of a large number of samples, oxidant consumption, long time, etc., and achieve the effect of retaining chemical integrity, reducing complexity, and less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The enzymatic hydrolysis and oxidative cleavage of the present embodiment are combined to prepare the method for mogrosolic alcohol and 11-oxidative-mogrosolic alcohol, comprising the following steps:
[0039] Step 1: Enzymatic hydrolysis of Mogroside V
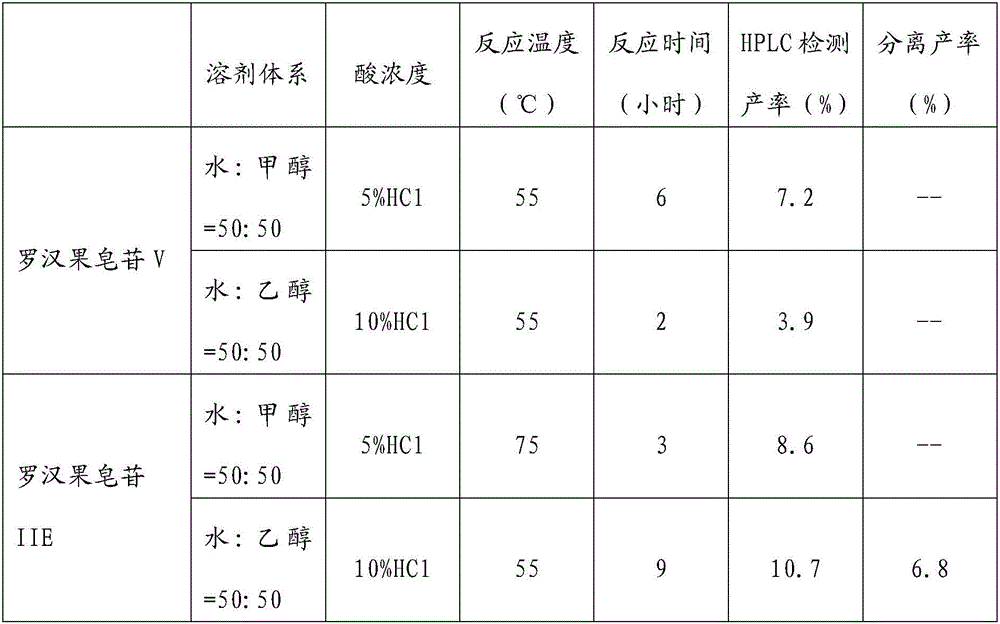
[0040] Take 6 g of hydrolase, dissolve it in 600 mL of water, take the supernatant, adjust pH=2 with acetic acid to obtain an enzymatic hydrolysis solution; take 6 g of Mogroside V, dissolve in 50 mL of the above enzymatic hydrolysis solution, react for 24 hours, and add ethanol to terminate the reaction, After filtration, 150 mL of mixed saponin solution was obtained;
[0041] Step 2: smith degradation
[0042] (1) Sodium Periodate Oxidative Cracking
[0043] In the mixed saponin solution obtained in step 1, 1 mL of acetic acid was added, and 50 mL of sodium periodate solution was slowly stirred under stirring, and the reaction was stirred at 20° C. in the dark to obtain a reaction solution after sodium periodate ox...
Embodiment 2
[0049] The enzymatic hydrolysis and oxidative cleavage of the present embodiment are combined to prepare the method for mogrosolic alcohol and 11-oxidative-mogrosolic alcohol, comprising the following steps:
[0050] Step 1: Enzymatic hydrolysis of Mogroside V
[0051] Take 10 g of hydrolase, dissolve it in 1000 mL of water, take the supernatant, adjust pH=4 with citric acid to obtain an enzymatic hydrolysis solution; take 100 g of commercial Mogroside V (50% content), use 1000 mL of the above enzymatic hydrolysis solution to dissolve, and react 18h, add ethanol to stop the reaction, after filtration, 2000mL mixed saponin solution was obtained;
[0052] Step 2: smith degradation
[0053] (1) Sodium Periodate Oxidative Cracking
[0054] In the mixed saponin solution obtained in step 1, 20 mL of acetic acid was added, and 400 mL of sodium periodate solution was slowly stirred under stirring, and the reaction was stirred at 50° C. in the dark to obtain a reaction solution after s...
Embodiment 3
[0060] The enzymatic hydrolysis and oxidative cleavage of the present embodiment are combined to prepare the method for mogrosolic alcohol and 11-oxidative-mogrosolic alcohol, comprising the following steps:
[0061] Step 1: Enzymatic hydrolysis of Mogroside V
[0062] Take 30 g of hydrolase, dissolve it in 3000 mL of water, take the supernatant, adjust pH=6 with phosphoric acid to obtain an enzymatic hydrolysis solution; take 500 g of commercial Mogroside V (50% content), dissolve it with 3000 mL of the above enzymatic hydrolysis solution, and react for 12 hours , adding ethanol to terminate the reaction, after filtration, to obtain 5000 mL of mixed saponin solution, the relative content of Mogroside V in the solution was found to be 38% by HPLC, the solvent was evaporated to dryness, and the reaction was dissolved with 3000 mL of enzymatic hydrolysis solution for 8 hours again, and ethanol was added to terminate the reaction, After filtration, 6000 mL of mixed saponin soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com