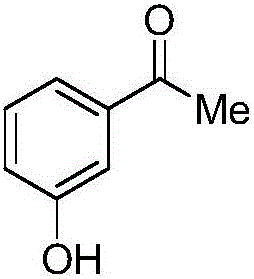
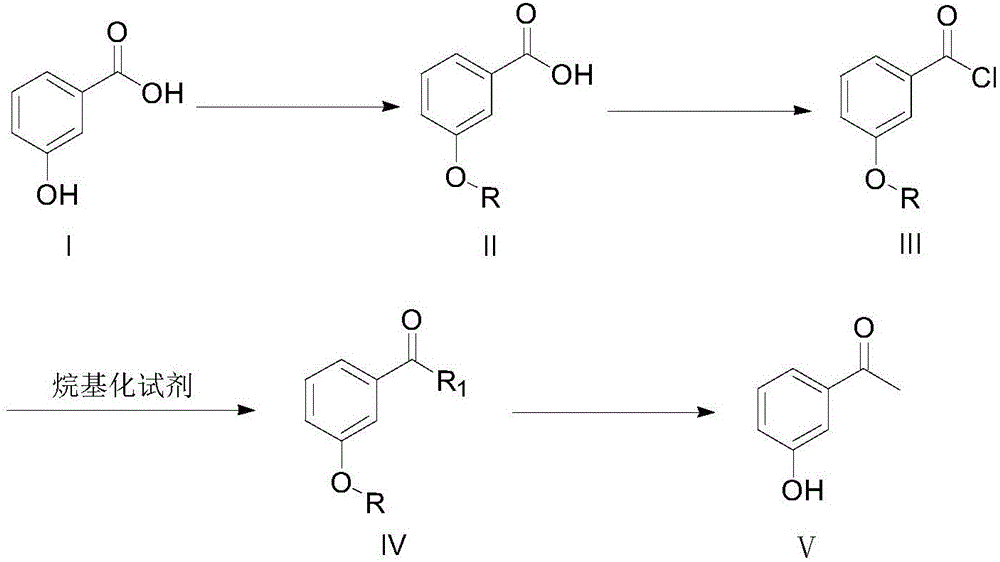
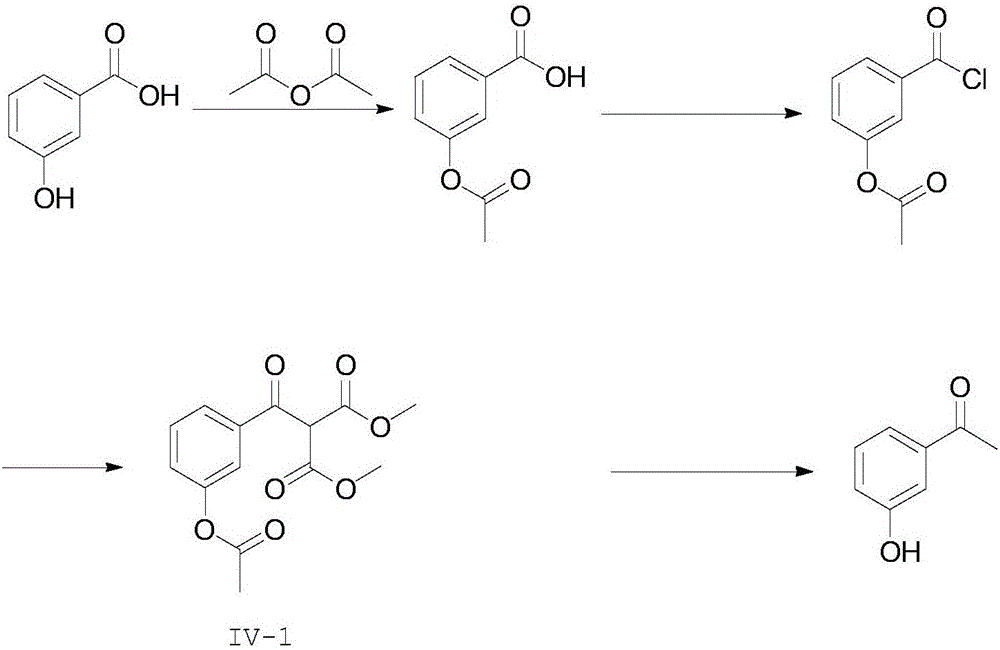
3-hydroxyacetophenone synthesis method
A technology for hydroxyacetophenone and a synthesis method, which is applied in the preparation of carbon-based compounds, chemical instruments and methods, and preparation of carbonyl compounds by condensation, etc., can solve the problems of large amount of sewage, serious environmental pollution and low yield in the production process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057]
[0058] Step (1): Add 50g of 3-hydroxybenzoic acid, 67g of acetic anhydride and 0.35g of concentrated sulfuric acid to the reaction vessel, heat up to 100°C under stirring and react for 30 minutes. After the reaction is over, distill under reduced pressure Removal of the solvent gave 64.5 g of 3-acetoxybenzoic acid (crude) as a white solid.
[0059] Step (2): Add 50 g of 3-acetoxybenzoic acid and 725 ml of toluene as a reaction solvent to the reaction vessel, then slowly add 56 g of thionyl chloride dropwise to the reaction system, and after the dropwise addition, heat up to the solvent After reacting at 100°C for 1 h, the solvent and unreacted thionyl chloride were removed by vacuum distillation to obtain 70.3 g of 3-acetoxybenzoyl chloride with a yield of 98% (crude product).
[0060] Step (3): Add 62.2 g of dimethyl malonate and 34.4 g of magnesium chloride to 600 ml of dichloromethane, slowly add triethylamine dropwise at an internal temperature of 5°C, and star...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com