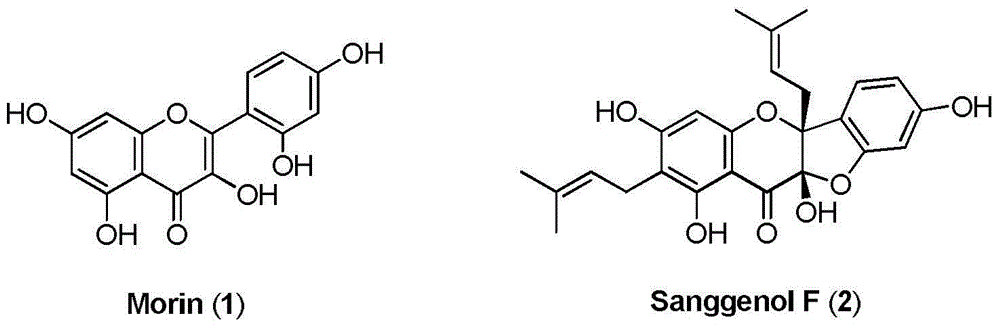
Total synthesis preparation method of natural product flavonoid compounds
A technology of flavonoids and natural products, applied in the direction of drug combination, organic chemistry, anti-toxin, etc., to achieve the effect of easy reaction, stable intermediates and easy access to raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
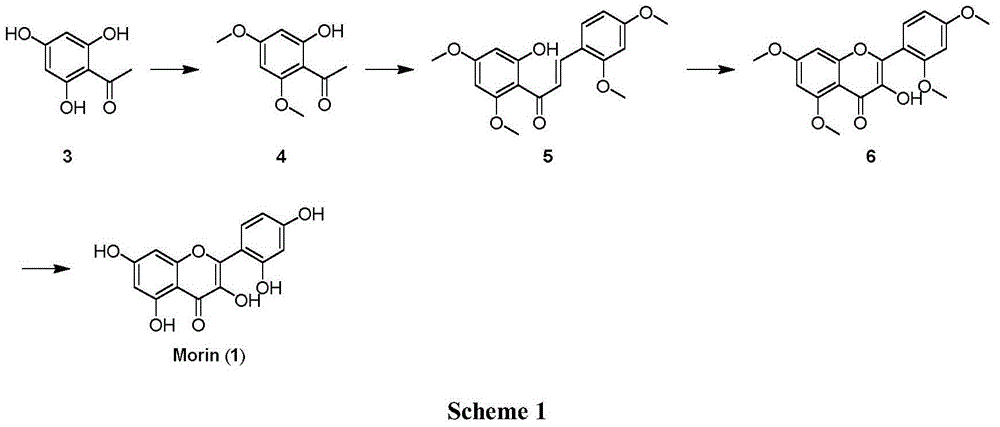
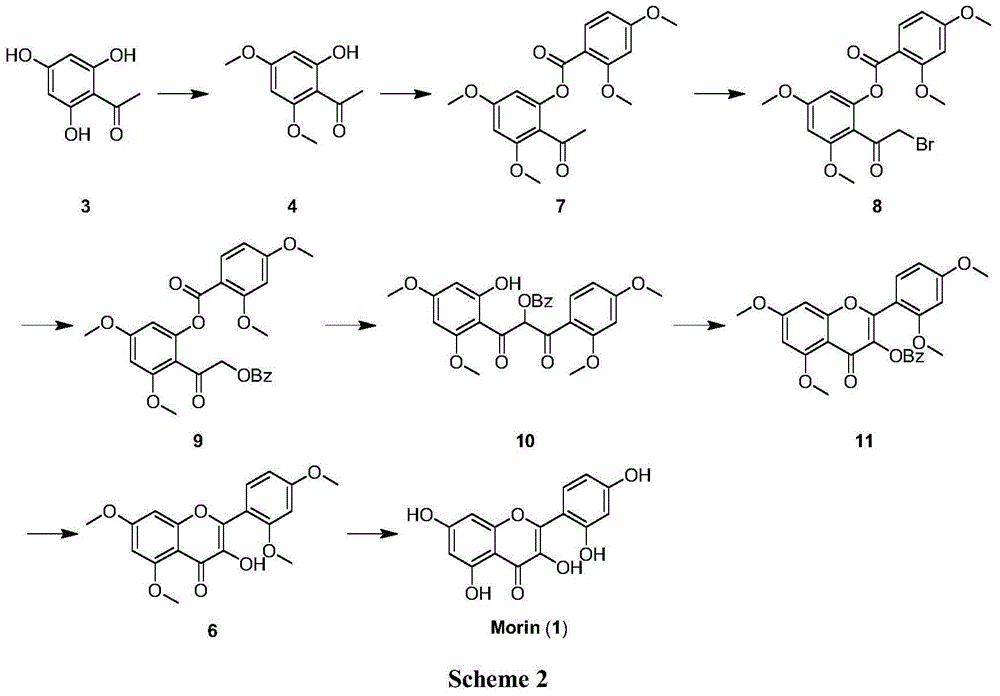
[0049] Embodiment 1 Synthetic 2-hydroxyl-4,6-dimethoxyacetophenone (4)
[0050] Weigh the substrate 2,4,6-trihydroxyacetophenone (10g, 59.50mmol) and K 2 CO 3 (16.5g, 119mmol) was placed in a 500mL eggplant-shaped bottle, added 150mL acetone and heated to reflux, and measured (CH 3 ) 2 SO 4 (3×3.76mL, 119mmol) was added into the reaction flask three times every two hours, and reacted for 6h. TLC monitoring (PE / EA 6:1) shows that raw material disappears substantially, in R f = 0.3 There is product formation. After filtration, the solvent was spun off to obtain 11.3 g of 4 as a light yellow solid, with a yield of 97%. mp 74-75℃.IR(neat):ν2970,2833,1617,1440,1157,895,597cm -1 . 1 H NMR (400MHz, CDCl 3 ):δ14.04(s,1H,OH),6.05(d,J=2.4Hz,1H,Ar-H),5.91(d,J=2.4Hz,1H,Ar-H),3.84(s,3H ,OCH 3 ),3.81(s,3H,OCH 3 ),2.60(s,3H,COCH 3 )...
Embodiment 2
[0051] Example 2 Synthesis of (E)-3-(2,4-dimethoxyphenyl)-1-(2-hydroxyl-4,6-dimethoxyphenyl)prop-2-en-1-one (5)
[0052] Weigh the above-mentioned 2-hydroxy-4,6-dimethoxyacetophenone (30mg, 0.15mmol) and 2,4-dimethoxybenzaldehyde (26mg, 0.15mmol) into a 10mL eggplant-shaped bottle, add 0.5 mL of ethanol was added dropwise to 50% aqueous KOH solution in an ice bath at 0°C, the ice bath was removed, and the reaction was carried out at room temperature for 2 days. TLC monitoring (PE / EA10:1) shows that raw material disappears substantially, in R f= 0.3 There is product formation. Adjust dilute hydrochloric acid to acidity, extract three times with ethyl acetate, wash three times with saturated sodium bicarbonate solution, three times with saturated brine, and dry over anhydrous sodium sulfate. Filtrate, spin off the solvent, and separate by column chromatography (PE / EA 10:1) to obtain 40 mg of 5 yellow solids with a yield of 76%. mp 132-133℃.IR(neat):ν2923,2366,1619,1219,1060,...
Embodiment 3
[0053] Example 3 Synthesis of 2-(2,4-dimethoxyphenyl)-3-hydroxyl-5,7-dimethoxy-4H-benzopyran-4-one (6)
[0054] Weigh substrate 5 (17mg, 0.05mmol) and dissolve in 0.25mL CH 3 OH (5mL / mmol), 0.16mL 5.4% NaOH aqueous solution (3.2mL / mmol) was added dropwise at 0°C in an ice bath, and then 0.02mL 30% H 2 o 2 (0.37 mL / mmol). The reaction solution was reacted at 0°C for 3h, and gradually raised to 40°C for 16h. TLC monitoring (PE / EA 1:1) shows that raw material disappears substantially, in R f = 0.23 there is product formation. Cool to room temperature, acidify with dilute hydrochloric acid, extract three times with ethyl acetate, wash three times with saturated aqueous sodium bicarbonate solution, three times with saturated brine, and dry over anhydrous sodium sulfate. Filtration, spin off the solvent, and column chromatography (PE / EA 1:1), the product 6 was obtained as light yellow solid 4mg, yield 23%. mp:156-157℃.IR(neat):ν3191,2920,1620,1412,1023,816,726cm -1 . 1 H NMR...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com