Water-soluble amino acid segmented copolymer, and preparation method and application thereof
A technology of block copolymers and amino acids, applied in the field of polyamino acids, can solve the problems of the complexity of the preparation process, change the assembly and morphology of nano-drugs, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
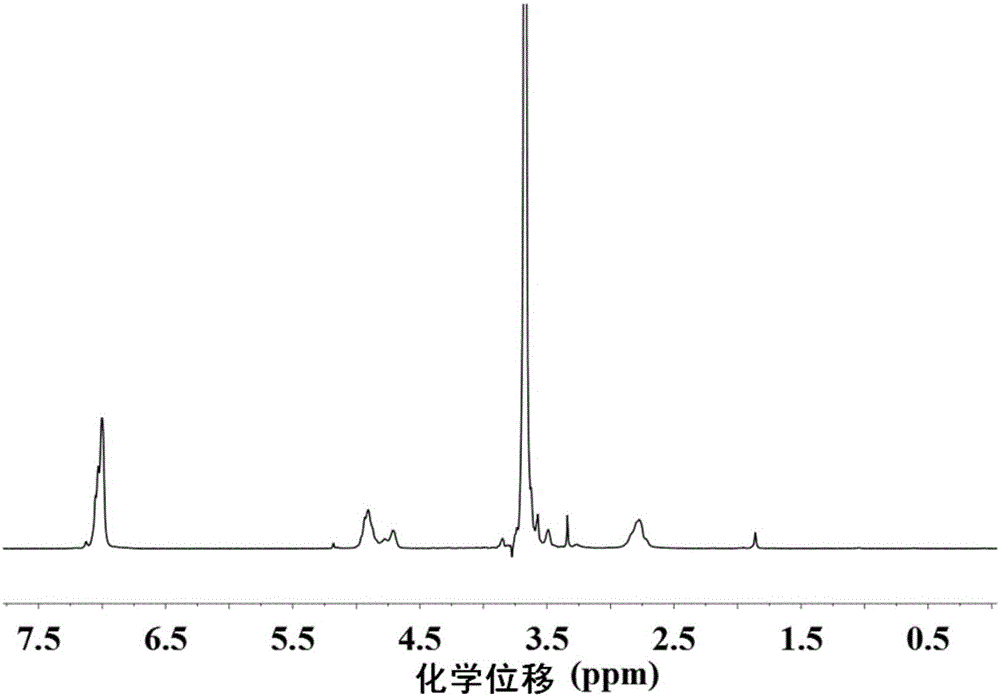
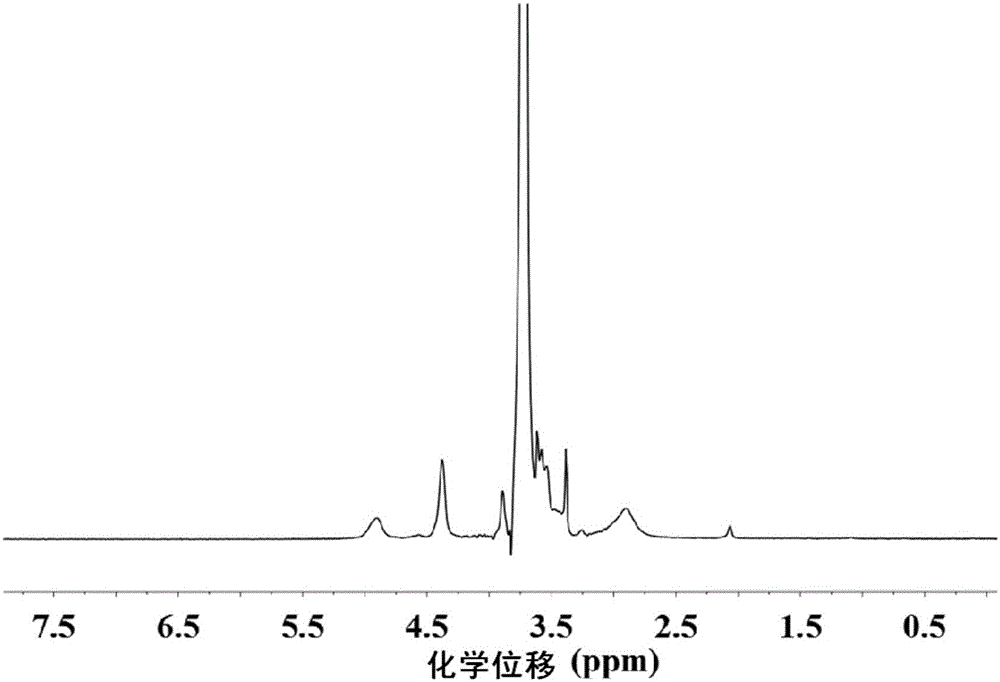
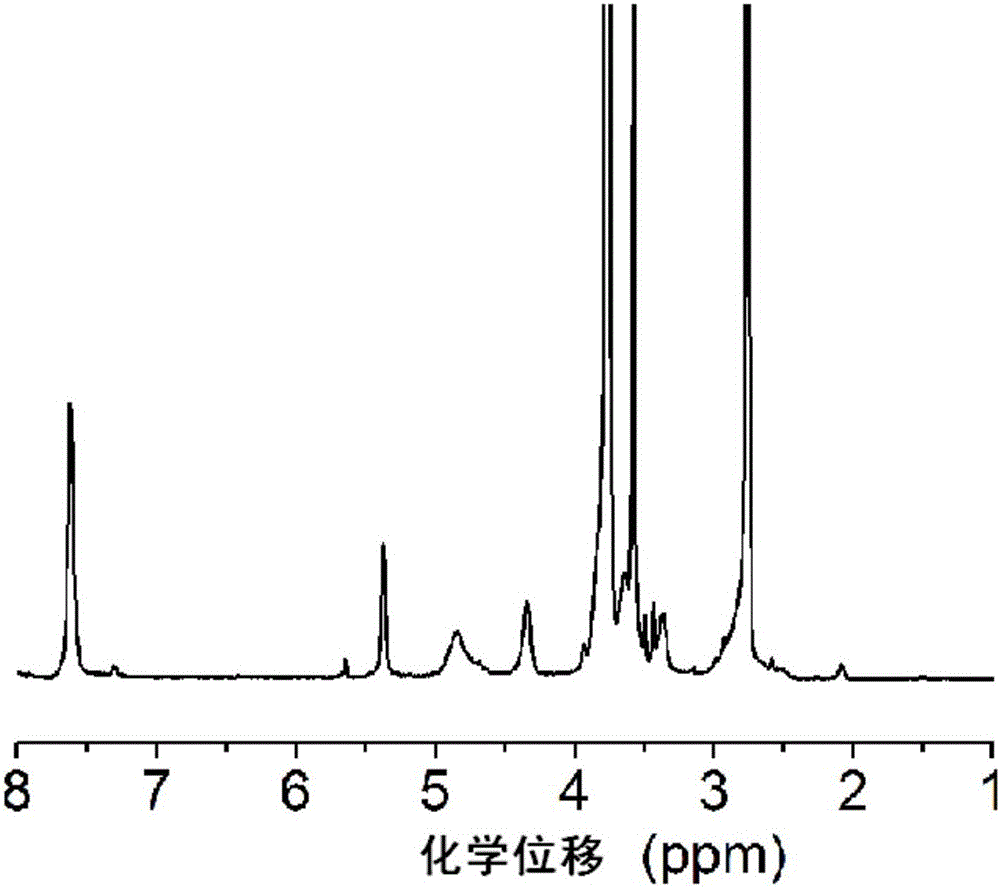
Image
Examples
preparation example Construction
[0081] The invention provides a method for preparing a water-soluble amino acid block copolymer, comprising: reacting a first block copolymer having a structure of formula III with ethanolamine to obtain an amino acid block copolymer having a structure of formula I;
[0082]
[0083] In formula III and formula I, R 1 selected from hydrogen, alkyl or substituted alkyl; R 2 selected from -NH- or -R 5 (CH 2 ) r NH-, where, R 5 is -O-, -OCONH-, -OCO-, -NHCOO- or -NHCO-, 1≤r≤10; R 4 Be selected from benzyl, cholesterol formyl, acetyl, cholic acid, deoxycholic acid or C4~C20 alkyl; 20≤n≤500; 5≤m≤200; in formula I, R 3 for hydrogen.
[0084]The present invention uses the first block copolymer having the structure of formula III as raw material, which includes polyaspartic acid segments with protective groups. The embodiment of the present invention provides the first block copolymer, and its preparation method is preferably as follows: a monoaminopolyethylene glycol compoun...
Embodiment 1
[0123] Add 5.00 g of a polyethylene glycol compound having a structure of formula V with a number average molecular weight of 5000 to the dry reaction flask, and remove water with 80 mL of anhydrous toluene at 130 ° C for 3 hours, and then vacuum dry the remaining toluene; the obtained solid was dissolved in 50 mL of dry N,N-dimethylformamide to obtain the first solution; 3.50 g of γ-benzyl-L-aspartic acid ester-N-internal carboxylic acid anhydride was dissolved in 40 mL of dry N,N-dimethylformamide to obtain a second solution. In a nitrogen atmosphere, the first solution and the second solution were mixed, stirred and reacted at room temperature under nitrogen protection for 48 h; then the temperature was raised to 35° C., and 10 mL of acetic anhydride was added to continue the reaction for 24 h. After the reaction, most of the N,N-dimethylformamide and unreacted acetic anhydride were sucked off under reduced pressure, then settled with diethyl ether, suction filtered, and dr...
Embodiment 2
[0129] In a dry round bottom flask, add 15.0g benzyl alcohol and 33.0g carbonyldiimidazole, add 100mL anhydrous dichloromethane to dissolve, and react at room temperature for 12h. After the reaction was completed, 500 mL of ethyl acetate was added to the system for dilution, and the organic layer was washed with distilled water and saturated brine successively, and then the organic layer was washed with anhydrous MgSO 4 Let dry overnight. The organic solvent was removed under reduced pressure to finally obtain 10.0 g of Bn-CDI.
[0130] In order to obtain the macromolecular material modified by benzyloxycarbonyl, add the block copolymer with formula I-a structure, Bn-CDI (145mg) and DMAP (105mg) prepared in 1.00g embodiment 1 in the dry reaction bottle, vacuumize 12h. Then 10 mL of dry N,N-dimethylformamide was added to dissolve, and the reaction was stirred at 50° C. for 12 h under nitrogen protection. After the reaction is finished, settle with excess ether, wash, filter ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| radius | aaaaa | aaaaa |
| radius | aaaaa | aaaaa |
| radius | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com