Method of partial vulcanization to improve oxygen evolution electrode performance of metal hydroxide
A technology of oxygen evolution electrode and hydroxide, which is applied in the direction of chemical instruments and methods, electrodes, chemical/physical processes, etc., can solve the problems of low catalytic activity and poor stability of oxygen evolution electrode, and improve intrinsic activity and stability performance, excellent oxygen evolution catalytic activity and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
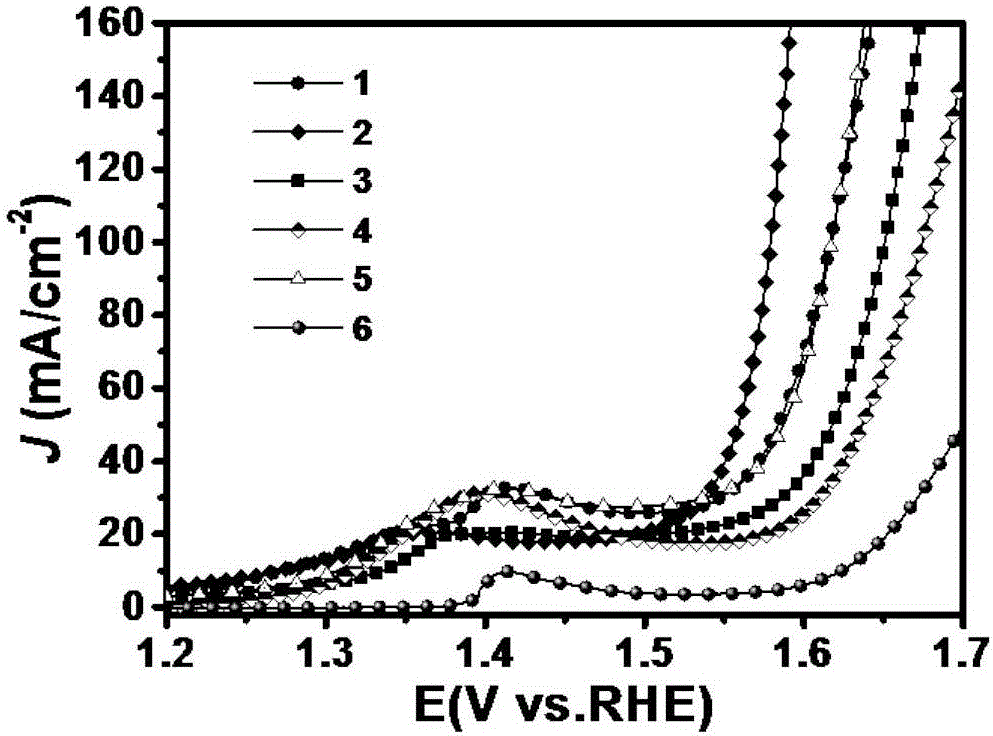
Embodiment 1
[0039] (1) Pretreatment of substrate
[0040] First put the nickel substrate into the lye and ultrasonically vibrate for 15 minutes for chemical degreasing, wherein the lye is a mixed aqueous solution of 45g / L sodium sulfate, 45g / L sodium carbonate and 45g / L sodium chloride, and then put into absolute ethanol Medium ultrasonic treatment for 15 minutes, and finally rinse with deionized water for later use;
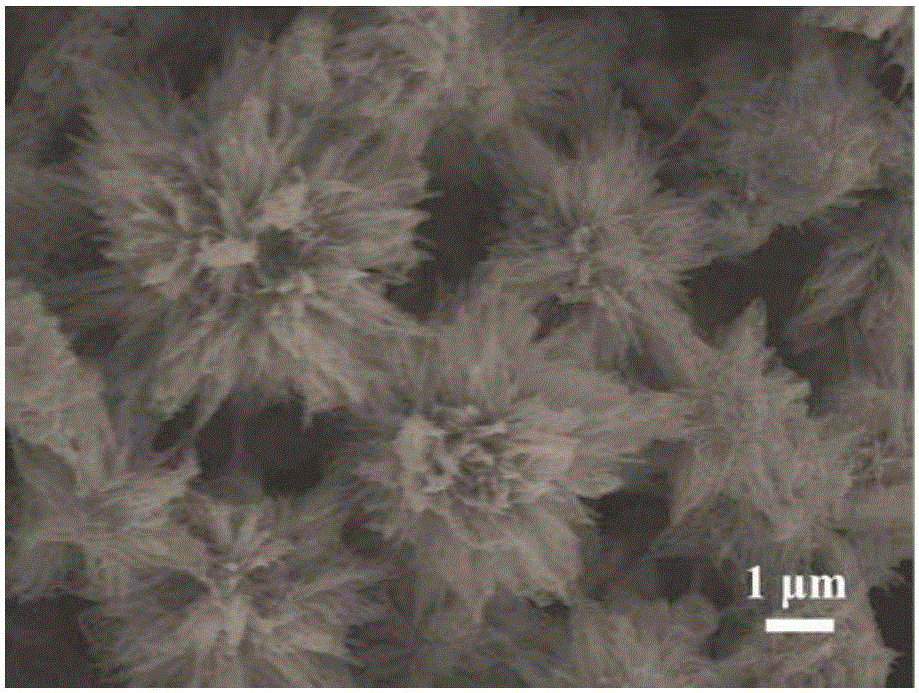
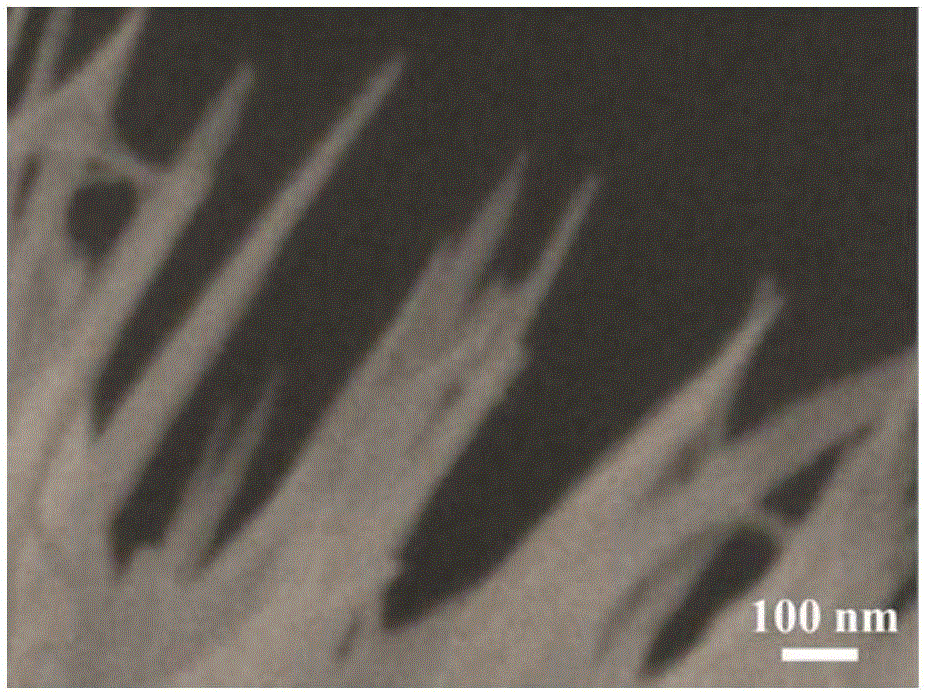
[0041] (2) Preparation of nano flower-shaped nickel-cobalt hydroxide electrode
[0042] Using deionized water as a solvent, prepare a mixed salt solution containing nickel nitrate and cobalt nitrate, wherein the molar concentration of nickel nitrate is 0.01 mol / liter, the molar concentration of cobalt nitrate is 0.02 mol / liter, and the molar concentration of urea is 0.05 mol / liter liter, the molar concentration of ammonium fluoride is 0.005 mol / liter; the base of step (1) pretreatment is put into the reactor that contains mixed salt solution, reacts 10 hours at the tempera...
Embodiment 2
[0052] Step (1) is the same as step (1) in Example 1;
[0053] (2) Preparation of nano-flower-like nickel-iron hydroxide electrode
[0054] Using deionized water as a solvent, prepare a mixed salt solution containing nickel nitrate and iron nitrate, wherein the molar concentration of nickel nitrate is 0.1 mol / liter, the molar concentration of ferric nitrate is 0.2 mol / liter, and the molar concentration of urea is 0.1 mol / liter liter, the molar concentration of ammonium fluoride is 0.02 mol / liter; the base of step (1) pretreatment is put into the reactor that contains mixed salt solution, reacts 5 hours at the temperature of 60 ℃; After the reaction finishes, use Rinse it with deionized water and place it in an inert atmosphere at a temperature of 40°C for 24 hours to dry it, then cool it to room temperature and take it out to prepare a nano-flower-shaped nickel-iron hydroxide electrode;
[0055] (3) Preparation of nickel-ironium sulfide oxygen evolution electrode
[0056] Th...
Embodiment 3
[0058] Step (1) is the same as step (1) in Example 1;
[0059] (2) Preparation of nanoflower-like iron-cobalt hydroxide electrode
[0060] Using deionized water as a solvent, prepare a mixed salt solution containing ferric nitrate and cobalt nitrate, wherein the molar concentration of ferric nitrate is 0.05 mol / liter, the molar concentration of cobalt nitrate is 0.02 mol / liter, and the molar concentration of urea is 0.05 mol / liter liter, the molar concentration of ammonium fluoride is 0.01 mol / liter; the base of step (1) pretreatment is put into the reactor that contains mixed salt solution, reacts 5 hours under the temperature of 200 ℃; After the reaction finishes, use Rinse it with deionized water and place it in an inert atmosphere at a temperature of 100°C for 2 hours to dry it, then cool it to room temperature and take it out to prepare a nano flower-shaped iron-cobalt hydroxide electrode;
[0061] (3) Preparation of Hydroxyl Iron Cobalt Sulfide Oxygen Evolution Electrod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com