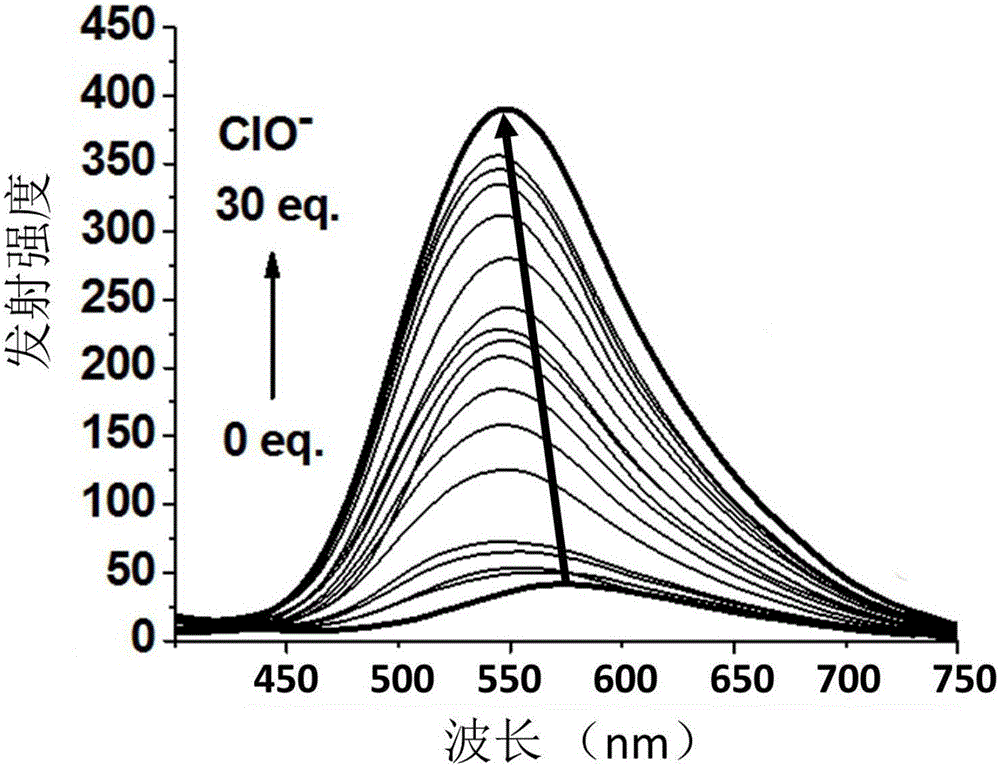
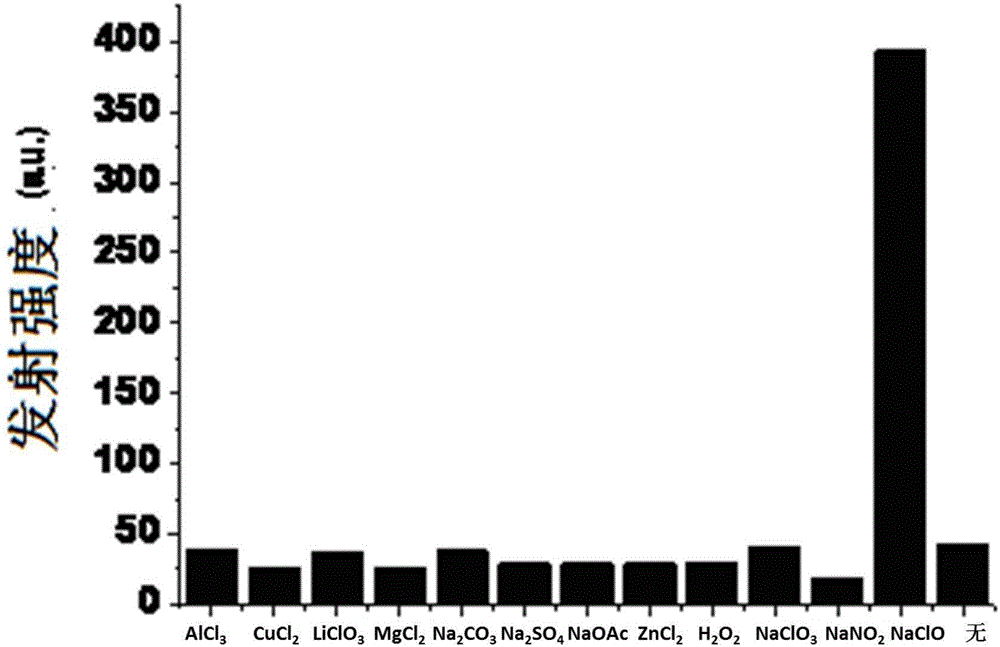
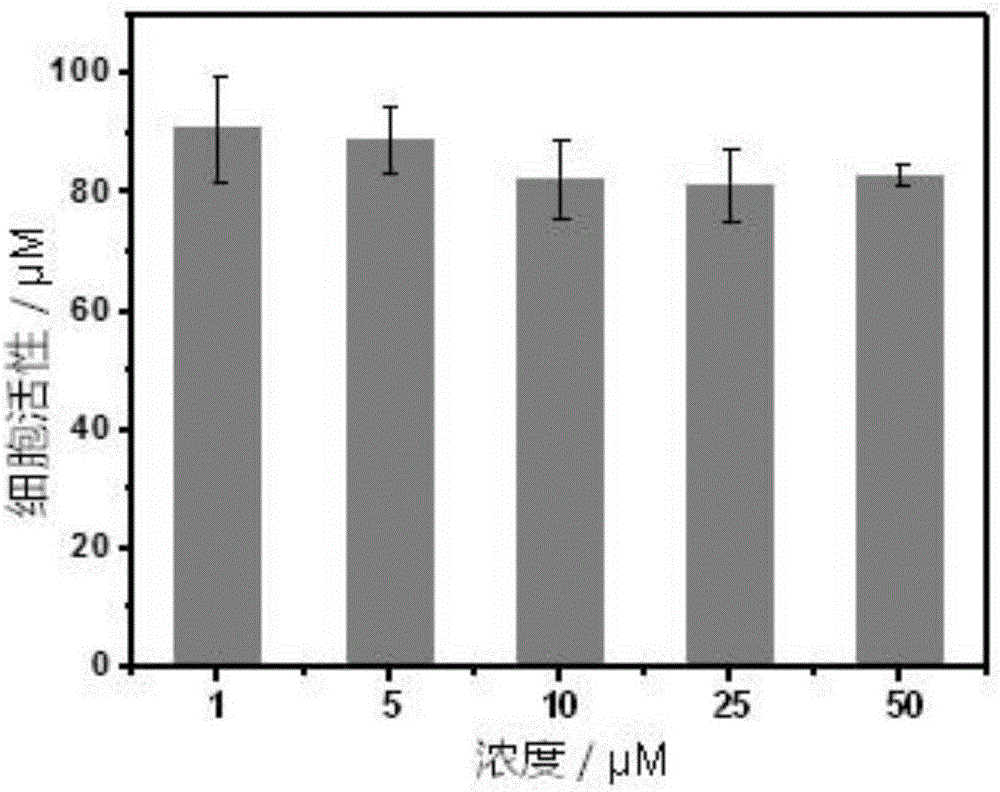
Phosphorescent iridium complex probe having mitochondrial targeting function as well as preparation and application thereof
A technology of iridium complexes and mitochondria, which is applied in the direction of indium organic compounds, platinum group organic compounds, fluorescence/phosphorescence, etc., can solve the problems of harm to organisms, and achieve high selectivity, low biological toxicity, and fast response.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: when n=2, for , the preparation of probe Ir1:
[0029] The synthetic route is as follows:
[0030]
[0031] Preparation of compound 2a: 51 mmol of potassium hydroxide and 10.2 mmol of 2-pyridylbenzimidazole were added to a reaction flask, and 20 ml of ionic liquid was added. After the mixture was pre-stirred for 5 minutes, 8ml of 1,6-dibromohexane was added, and the reaction was stirred at room temperature for 5h. After the reaction was completed, it was extracted three times with ether, the oil phases were combined, and the solvent was removed by a rotary evaporator. The obtained crude product was separated by column chromatography, and the eluent was petroleum ether:ethyl acetate=3:1, and finally 2.2 g of a colorless oily liquid was obtained, with a yield of 60%. 1 H NMR (400MHz, CDCl 3 ): δ=8.70(d, J=4.80Hz, 1H), 8.41(d, J=7.97Hz, 1H), 7.88-7.33(m, 2H), 7.46-7.44(m, 1H), 7.37-7.30( m, 3H), 4.84(t, J=7.6Hz, 2H), 3.38(t, J=6.8Hz, 2H), 1.95-1.88...
Embodiment 2
[0045] Embodiment 2: when n=1, for When, the preparation of probe Ir2:
[0046] The synthetic route is as follows:
[0047]
[0048] Preparation of compound 2b: 51 mmol of potassium hydroxide and 10.2 mmol of 2-pyridylbenzimidazole were added to a reaction flask, and 20 ml of ionic liquid was added. After the mixture was pre-stirred for 5 minutes, 8ml of 1,4-dibromobutane was added, and the reaction was stirred at room temperature for 5h. After the reaction was completed, it was extracted three times with ether, the oil phases were combined, and the solvent was removed by a rotary evaporator. The obtained crude product was separated by column chromatography, and the eluent was petroleum ether: ethyl acetate = 3:1, and finally 2.2 g of a colorless oily liquid was obtained, with a yield of 62%. 1 H NMR (400MHz, CDCl 3 ): δ=8.70(d, J=4.80Hz, 1H), 8.41(d, J=7.97Hz, 1H), 7.88-7.33(m, 2H), 7.46-7.44(m, 1H), 7.37-7.30( m, 3H), 4.86(t, J=7.6Hz, 2H), 3.39(t, J=6.8Hz, 2H), 1....
Embodiment 3
[0061] Embodiment 3: when n=2, for When, the preparation of probe Ir3:
[0062] The synthetic route is as follows:
[0063]
[0064] Synthesis of p-formylbenzoquinoline (1c): Add 2-chloroquinoline (818mg, 5.0mmol), p-formylphenylboronic acid (750mg, 5.0mmol) and tetrakis (triphenylphosphine) palladium catalyst in a 250mL two-neck flask (200mg). The system is sealed, deoxygenated and filled with nitrogen for protection. Then, the solvent toluene (30 mL), ethanol (10 mL), and saturated aqueous sodium carbonate solution (10 mL) were added by syringe after deoxygenating with nitrogen for half an hour. During the reaction, the system was protected from light, and the reaction was refluxed at 80° C. for 15 hours. After the reaction was finished, the system was cooled to room temperature. The reaction solution was extracted three times with dichloromethane / water, the organic phase was collected, concentrated, and spotted by TLC to confirm the product point. The crude prod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com