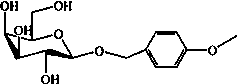
High-temperature-resistance sustained-release glycoside-type anisealcohol, and enzymatic preparation method and application thereof
A slow-release glycoside type, anisol technology, applied in application, sugar derivatives, food science and other directions, can solve problems such as unstable physical and chemical properties, and achieve high heat loss rate, good water solubility, and good thermal stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] In the present invention, the recombinant β-galactosidase can be prepared by the method reported in the existing research results in this field. It can also be prepared by the following method:
[0059] Main materials and reagents: restriction endonuclease, T 4 DNA ligase, Ex Taq enzyme, restriction endonuclease EcoRI, NotI, SalI, etc. were purchased from Dalian Bao Biotechnology Engineering Co., Ltd., and agarose gel recovery kit and plasmid mini-extraction kit were purchased from Beijing Tiangen Biochemical Co., Ltd. Technology Co., Ltd., and the primers were synthesized by Shanghai Jierui Bioengineering Co., Ltd. E.coli DH5α, E.coli BL21(DE3), pET32a(+), pPIC9k vector and yeast strain GS115 were purchased from Invitrogene; the cloning vector pMD18-T was purchased from Dalian Bao Biotechnology Engineering Co., Ltd.
[0060] Lactobacillus plantarum can be used conventionally used Lactobacillus plantarum. For the convenience of description, this embodiment adopts Lact...
Embodiment 2
[0091] The synthesis of embodiment 2 compound anisyl alcohol-beta-galactoside
[0092] This embodiment provides its synthetic route diagram at the same time and sees attached Figure 9 shown.
[0093] S1. Fully dissolve 1mmol D-lactose in 0.2mL disodium hydrogen phosphate-sodium dihydrogen phosphate buffer solution (50mM, pH5.0), add 1.8mL anisyl alcohol, and mix well;
[0094] S2. Add β-galactosidase 6U / mL to the system obtained in step S1, start the reaction, and shake the reaction at 40°C for 96 hours to prepare anisyl alcohol-β-galactoside; the β-galactosidase in this example was purchased from SIGAMA Reagent Company.
[0095] S3. Add an equal volume of methanol to the reaction system in step S2 to terminate the reaction, boil the reaction solution in boiling water for 10 minutes to inactivate the enzyme, filter the enzyme and the unreacted substrate completely, add a small amount of water to the filtrate, and add an equal volume of ether Multiple extractions were perfo...
Embodiment 3
[0097] The synthesis of embodiment 3 compound anisyl alcohol-beta-galactoside
[0098] This embodiment provides its synthetic route diagram at the same time and sees attached Figure 9 shown.
[0099] S1. Fully dissolve 1mmol D-lactose in 0.2mL disodium hydrogen phosphate-sodium dihydrogen phosphate buffer solution (50mM, pH5.0), add 1.8mL anisyl alcohol, and mix well;
[0100] S2. Add β-galactosidase 6U / mL to the system obtained in step S1, start the reaction, shake the reaction at 40°C for 96 hours, and prepare anisyl alcohol-β-galactoside; this example uses recombinant β-galactosidase (Common market purchase).
[0101] S3. Add an equal volume of methanol to the reaction system in step S2 to terminate the reaction, boil the reaction solution in boiling water for 10 minutes to inactivate the enzyme, filter the enzyme and the unreacted substrate completely, add a small amount of water to the filtrate, and add an equal volume of ether Multiple extractions were performed to r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| flash point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com