Nutrient composition and application thereof in preparation of medicines for treating aplastic anemia
A nutritional composition and vitamin technology, applied in the field of life sciences, can solve the problems of DNA repair ability reduction, failure to correct, hematopoietic microenvironment support function defects, etc., and achieve the effect of treating aplastic anemia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1: The mass (mg) ratio of each component of the nutritional composition of the present invention is as follows: lysine 200-1200, methionine 100-1600, phenylalanine 150-1400, threonine 50-600, Tryptophan 50~400, Arginine 200~1500, Histidine 100~1000, Glycine 100~600, Aspartic Acid 100~600, Leucine 100~600, Isoleucine 100~600, Valine Amino acid 100~800, Serine 100~400, Glutamine 200~600, Taurine 100~500, Orotic acid 100~300, Nucleotide 200~1200, Vitamin A 0.2~0.6, Vitamin D 0.002~0.006 , Vitamin B 2 1~4, vitamin B 6 1~4, vitamin B 12 0.001~0.006, niacin 5~20, folic acid 0.01~1.2, vitamin C 50~150, iron 2~10, zinc 2~10, manganese 2~10, copper 0.5~2, selenium 0.01~0.1, chromium 0.01~0.02 , potassium 10~300, calcium 100~300, magnesium 100~200, inositol 50~60, soybean lecithin 100~2500.
Embodiment 2
[0053] Example 2: The mass (mg) ratio of each component is as follows: lysine 600, methionine 800, phenylalanine 700, threonine 300, tryptophan 200, arginine 760, histidine 500 , Glycine 300, Aspartic Acid 300, Leucine 300, Isoleucine 300, Valine 400, Serine 200, Glutamine 300, Taurine 500, Orotic Acid 300, Nucleotide 400, Vitamin A0.6, vitamin D0.006, vitamin B 2 2. Vitamin B 6 2. Vitamin B 12 0.004, niacin 12, folic acid 0.9, vitamin C100, iron 10, zinc 10, manganese 8, copper 2, selenium 0.1, chromium 0.02, potassium 300, calcium 300, magnesium 200, inositol 60, soybean lecithin 1200.
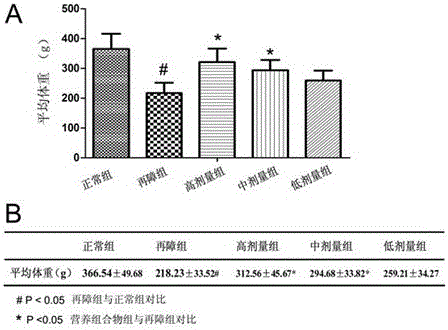
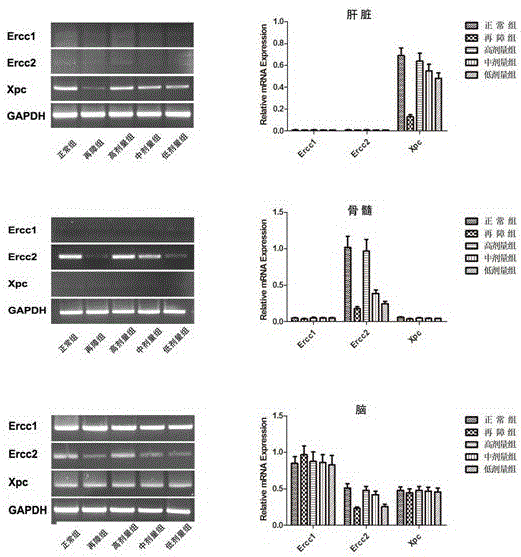
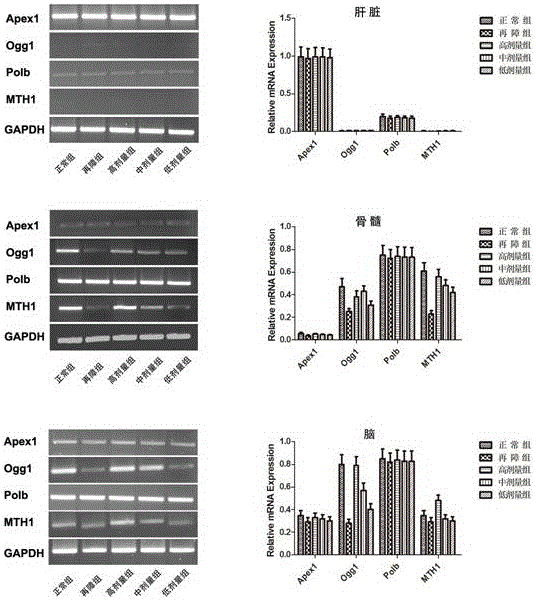
[0054] The nutritional composition can be used in the preparation of medicines for treating aplastic anemia.
[0055] The sources of raw materials are shown in Table 1:
[0056] Table 1
[0057]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com