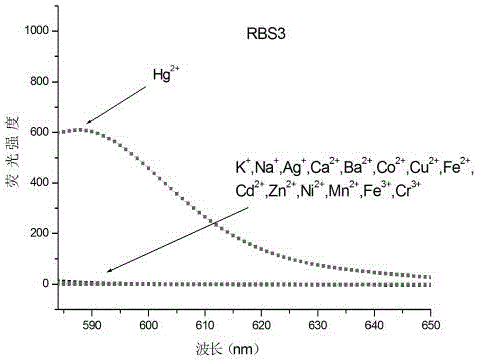
Substituted Rhodamine B acylthiosemicarbazide fluorescence probe compound and preparation method and application thereof
A technique for fluorescent probes and hydrazide compounds is applied in the field of substituted rhodamine B amidothiourea fluorescent probe compounds and their preparation, and can solve the problems of high requirements for instruments and equipment, high price, and no continuous detection allowed. Achieve good anti-interference ability, good selectivity and good sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Synthesis of rhodamine B hydrazide compound
[0033] Take 1.443 g (3 mmol) of Rhodamine B and transfer it into a 100 mL round bottom flask, dissolve it with 50 mL of ethanol, then pipette 3 mL (80 wt%, 20 mmol) of hydrazine hydrate aqueous solution with a pipette gun, and the reaction system is now Dark wine red, reflux reaction in an oil bath at 85°C, monitor the progress of the reaction by thin-layer chromatography (TLC), the developer is a mixed solution of petroleum ether and ethyl acetate with a volume ratio of 1:1, and spot the plate after 6 hours of reaction It was found that the raw material spots basically disappeared on the thin-layer plate, and the rhodamine hydrazide product spots appeared at the position of about 0.5 in the ratio shift value. After the reaction was quenched, the system was extracted with ethyl acetate, and the organic phase was collected and then distilled under reduced pressure to obtain 1.2537 g of rhodamine hydrazide compound, with a yie...
Embodiment 2
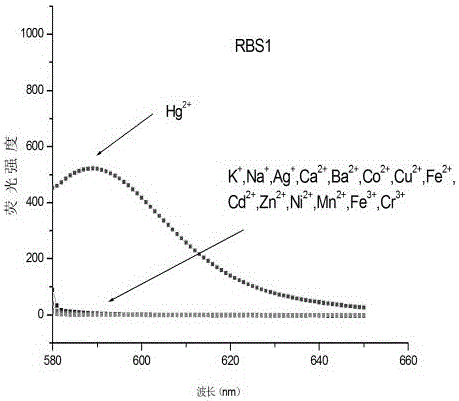
[0035] Synthesis of o-chlororhodamine probe RBS1
[0036] Take 0.1260 g (0.75 mmol) of 2-chlorophenylisothiocyanate and 0.2292 g (0.5 mmol) of rhodamine hydrazide compound and transfer them to a 25 mL round-bottomed flask, then add 5 mL of dry isopropanol to Magnetically stir the reflux reaction in an oil bath at 85°C, and monitor the reaction by TLC after reacting for 5 minutes. The developer is a mixed solution of petroleum ether and ethyl acetate with a volume ratio of 1:1. After 10 hours, the raw material point disappears, and the reaction is stopped. , after the system was lowered to room temperature, the solvent was distilled off under reduced pressure and neutral alumina was added to its dry-mixed sample, and neutral alumina was used as the stationary phase for column chromatography (petroleum ether: ethyl acetate=10:2, v / v) Separation and purification, the product is white fluffy powder ortho-chlororhodamine probe RBS1 (yield 80%).
Embodiment 3
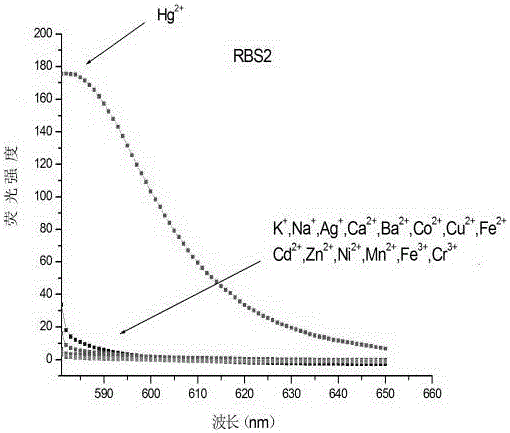
[0038] Synthesis of o-methoxyrhodamine probe RBS2
[0039] Take 0.1238 g (0.75 mmol) of 2-methoxyphenylisothiocyanate and 0.2292 g (0.5 mmol) of rhodamine hydrazide compound and transfer to a 25 mL round bottom flask, then add 5 mL of dry isopropanol , magnetically stirred and refluxed in an oil bath at 85°C. After 5 minutes of reaction, TLC was used to monitor the reaction. The developer was a mixed solution of petroleum ether and ethyl acetate with a volume ratio of 1:1. After 10 hours, the raw material point disappeared. After the reaction was stopped and the system was lowered to room temperature, the solvent was distilled off under reduced pressure and neutral alumina was added to dry mix the sample, and neutral alumina was used as the stationary phase for column chromatography (petroleum ether: ethyl acetate=10:2 , v / v) separation and purification, the product is white fluffy powder o-methoxyrhodamine probe RBS2 (yield 82%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com