Method for preparing nano oral protein drug delivery system and application thereof
A protein and drug technology, applied in the field of preparation of nanometer oral protein drug delivery system, can solve problems such as enhancing therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Example 1 Preparation of nanometer oral protein drug delivery system
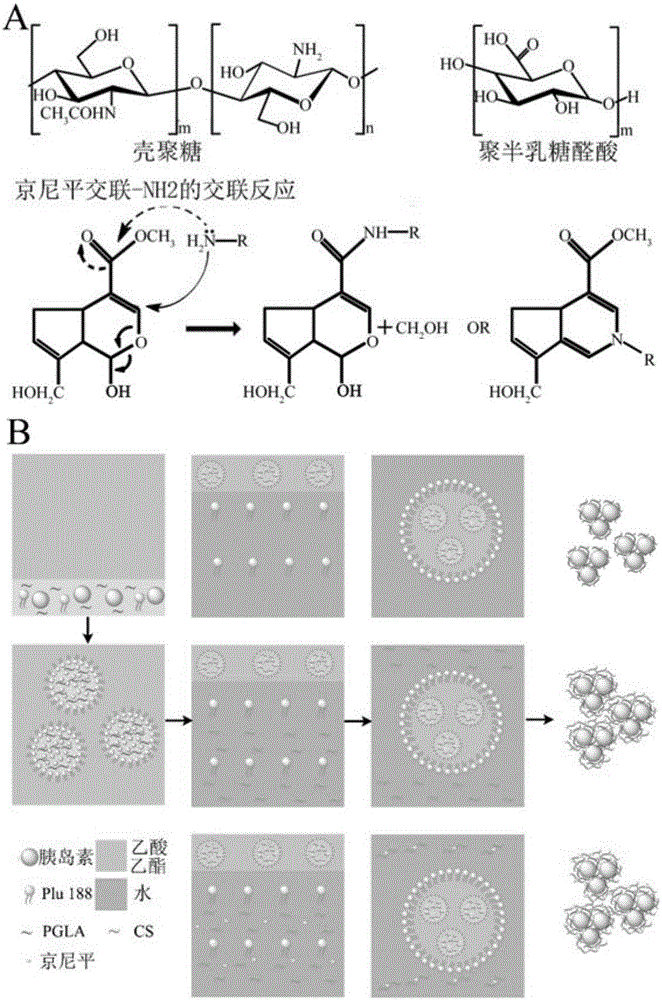
[0098] Synthesize nanoparticles (nano oral protein drug delivery system) by W / O / W double emulsification method, as attached figure 1 shown.
[0099] Specifically, the preparation method of the nano oral protein drug delivery system is as follows:
[0100] 1. Preparation of insulin-loaded PGLA nanoparticles (PGLA NP)
[0101] (1) Prepare the inner aqueous phase:
[0102] Add PGLA to 5% Pluronic 188, adjust to pH=4, incubate at 50°C for 30min, shake, dissolve for 24h, centrifuge to remove insoluble precipitate, take 200μl of the obtained solution, add 1mg bovine insulin to dissolve; the PGLA is mixed with 5% (w / v) Poloxamer 188 has a mass volume ratio of 10 mg / ml.
[0103] (2) Prepare PGLA NP with ethyl acetate as the oil phase and 20% (w / v) Pluronic 188 as the external aqueous phase solution:
[0104] Add the internal water phase of step (1) into 1ml of ethyl acetate, and ultrasonicate for 60s to...
Embodiment 2
[0114] The characterization of embodiment 2 nano oral protein drug delivery system
[0115] 1. Infrared spectroscopy detection (FTIR)
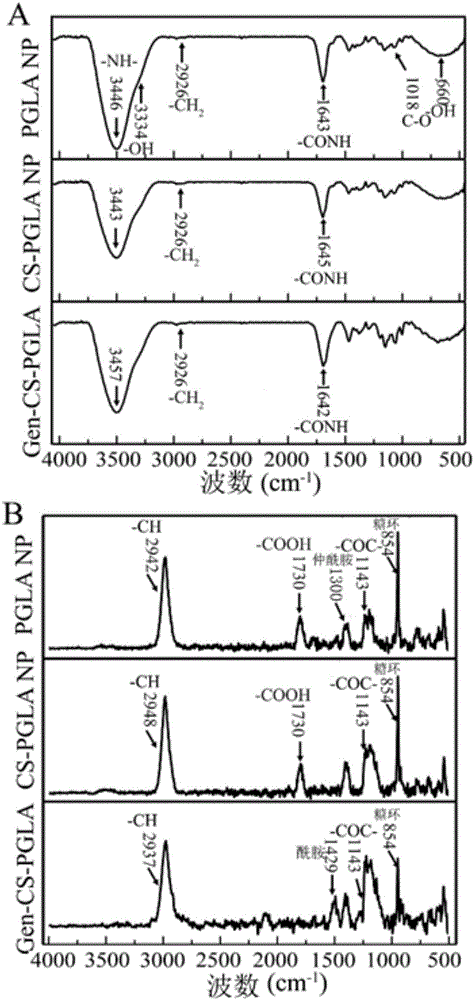
[0116] (1) The raw materials PGLA, chitosan, insulin, genipin and the three systems synthesized by the system were detected by infrared spectroscopy. Freeze-dry PGLA NP, CS-PGLA NP, and Gen-CS-PGLA NP, then put them into a mortar, add a certain amount of KBr, and grind the mixture evenly until the particle size is less than 2 μm, so as not to be affected by scattered light, then put it into a dry Machine drying. Press the mixture into a transparent sheet with a pressure of about 10 MPa on a hydraulic press, and use a German Bruker VERTEX 33 Fourier transform infrared spectrometer to measure it.
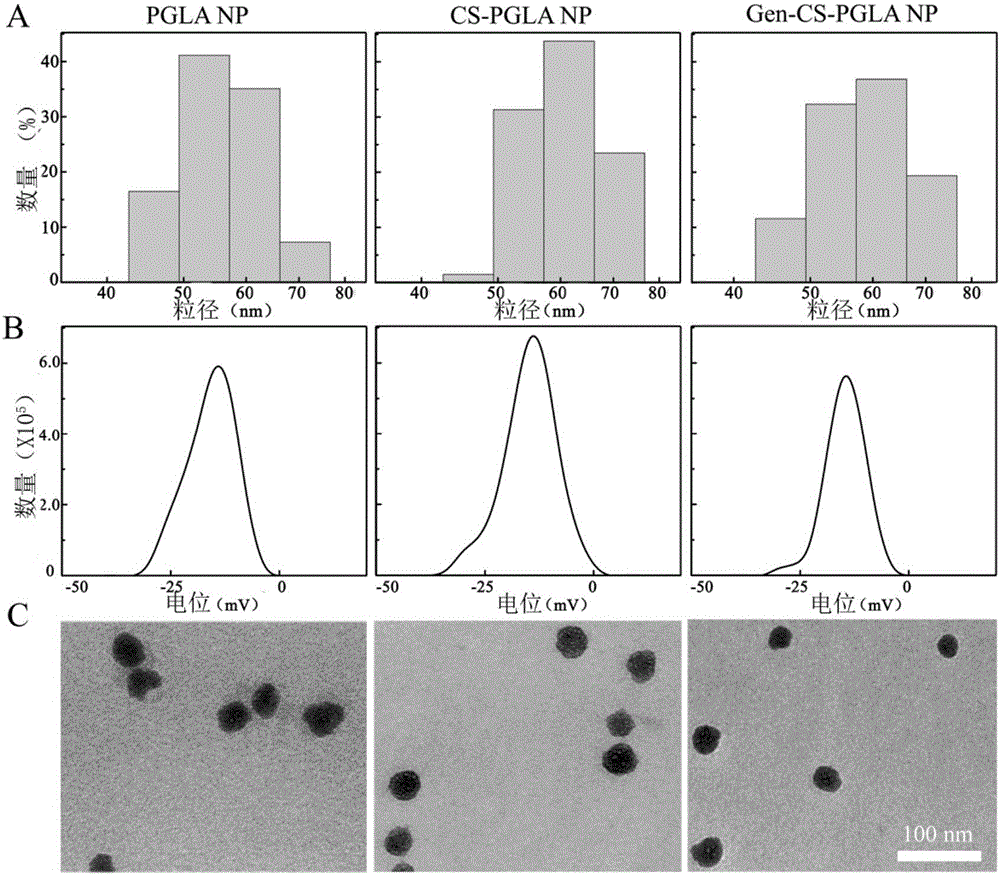
[0117] (2) Infrared spectroscopy analysis can explain to a certain extent the composite components of the synthesized nanosystems ( figure 2 A). The infrared spectra of PGLA NP, CS-PGLA NP and Gen-CS-PGLA NP show that the three are at 1100cm -1 ...
Embodiment 3
[0149] Example 3 Cell Experiment of Nano Oral Protein Drug Delivery System
[0150] 1. Cell culture
[0151] Caco-2 cell line was provided by Guangdong Pharmaceutical University. After the cells were proliferated and cultured to 80% in the culture flask, they were seeded on a 96-well plate at a density of 5000 / well, and cultured for 1 or 2 days for subsequent experiments. The cell culture conditions are: high-glucose DMEM medium containing 20% newborn calf serum, non-essential amino acids, 37°C constant temperature, 5.0% CO 2 .
[0152] 2. Cytotoxicity test of the delivery system
[0153] (1) Add different doses of the delivery system to the Caco-2 cells being cultured, and after 24 hours, use the MTT method to measure the cell survival rate. That is to inoculate Caco-2 cells on a 96-well culture plate, 5000 cells / well, after 24 hours of culture, aspirate the medium, add serum-free medium, and different concentrations of insulin delivery system, after 24 hours of culture...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com