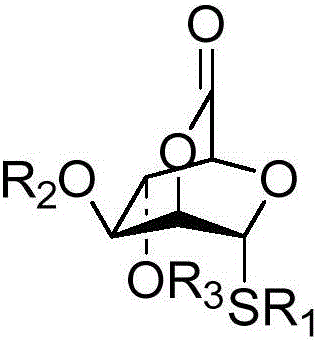
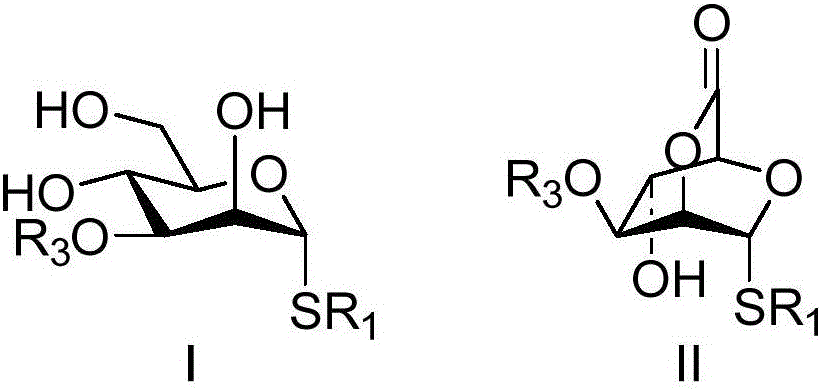
Bridged ring lactone compounds, a preparing method thereof and applications of the compounds in construction of beta-mannoside bonds
A technology of ester compounds and mannosidic bonds, applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve time-consuming problems and achieve simple operation, high stereoselectivity, and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
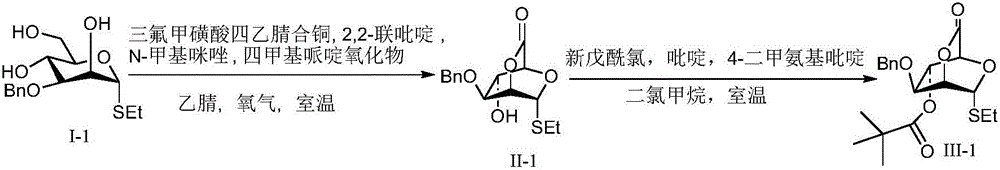
[0023] Taking the bridged lactone compound shown in the synthetic formula III-1 as an example, its synthetic route and specific synthetic method are as follows:
[0024]
[0025] 1. 1.1067g (3.52mmol) ethylthio-3-benzyl-1-thio-α-mannose shown in formula I-1, 397mg (1.056mmol) tetraacetonitrile copper trifluoromethanesulfonate, Add 54.9mg (0.352mmol) of 2,2-bipyridine and 15mL of acetonitrile into a 50mL round-bottomed flask, vacuum three times for oxygen; add 71.5mg (0.457mmol) of tetramethylpiperidine oxide, Dissolve N-methylimidazole in 1 mL of acetonitrile, put the resulting solution into a round-bottomed flask, react in the dark at room temperature for 10 hours in an oxygen atmosphere, filter, spin dry the solvent with a rotary evaporator, and measure the volume ratio of ethyl acetate to petroleum ether The mixed solution of 3:1 was used as the eluent for column chromatography separation to obtain the compound of formula II-1 with a yield of 82%, and the structural char...
Embodiment 2
[0028] Taking the bridged ring lactone compound shown in the synthetic formula III-2 as an example, its synthetic method is as follows:
[0029]
[0030] Step 1 of this embodiment is the same as step 1 of Embodiment 1. In step 2 of Example 1, the pivaloyl chloride used is replaced with equimolar naphthoyl chloride, and the reaction time is shortened to 2 hours. Other steps are the same as in Example 1 to obtain the bridged ring lactone shown in formula III-2 Compound, its yield is 85%, structural characterization data is: 1 H NMR (400MHz, CDCl 3 )δ8.17-8.10(m,2H),7.67-7.59(m,1H),7.54-7.45(m,2H),7.35-7.26(m,5H),5.36(d,J=2.8Hz,1H) ,5.27(dt,J=2.3,1.2Hz,1H),4.92–4.86(m,1H),4.74-4.62(m,2H),4.61(dd,J=2.2,0.7Hz,1H),4.21(s ,1H),2.78(q,J=7.4Hz,2H),1.34(t,J=7.4Hz,3H).; 13 C NMR (101MHz, CDCl 3 )δ166.71, 164.96, 136.41, 133.64, 130.07, 129.02, 128.54, 128.49, 128.18, 127.93, 80.52, 77.36, 77.32, 77.00, 76.68, 74.67, 74.19, 71.84, 71.37; Na + ])C 22 h 22 NaO 6 The theoretical...
Embodiment 3
[0032] Taking the bridged ring lactone compound shown in the synthetic formula III-3 as an example, its synthetic method is as follows:
[0033]
[0034] Step 1 of this embodiment is the same as step 1 of Embodiment 1. In step 2, 123.9mg (0.399mmol) of compound II-1 was azeotroped with toluene three times to remove water, then it was dissolved with 2.1mL of anhydrous dichloromethane under argon protection and stirring in an ice bath, and 104.5mg (0.478mmol) di-tert-butyl dicarbonate, 4.8mg (0.040mmol) 4-dimethylaminopyridine, after reacting at room temperature for 1 hour, the reaction was complete through TLC point plate detection, concentrated, column chromatography (eluent was Petroleum ether and ethyl acetate volume ratio is the mixed solution of 3:1), obtains the bridged ring lactone compound shown in formula III-3, and its productive rate is 86%, and structural characterization data is: 1 H NMR (400MHz, CDCl 3 )δ7.39-7.30 (m, 5H), 5.26 (d, J = 2.6Hz, 1H), 4.84 (d, J ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com