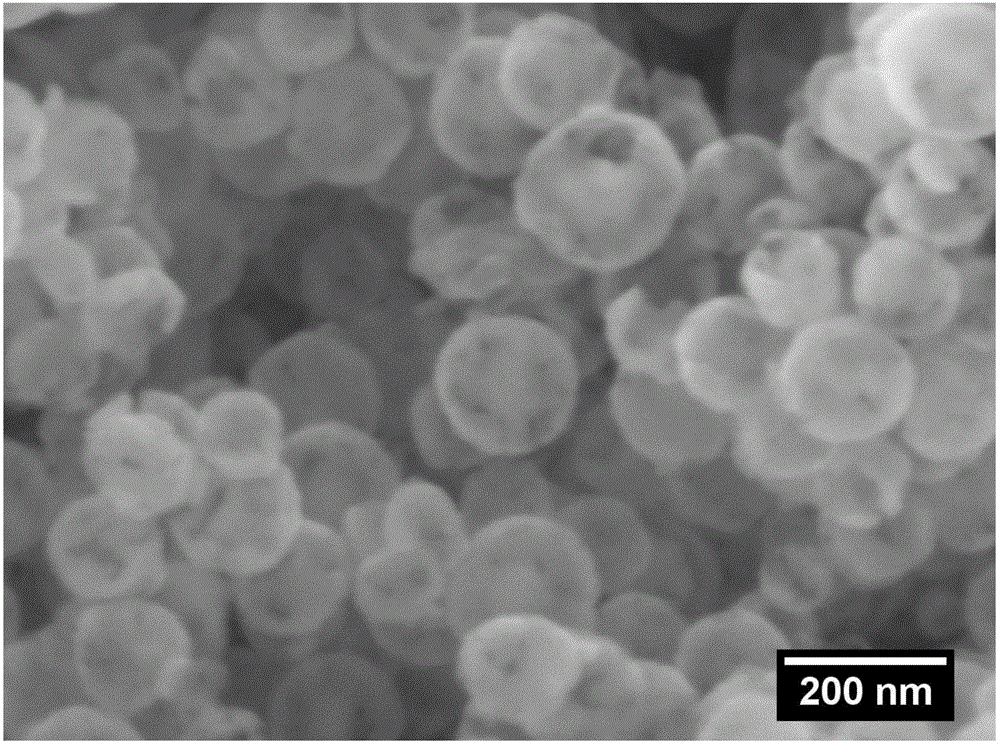
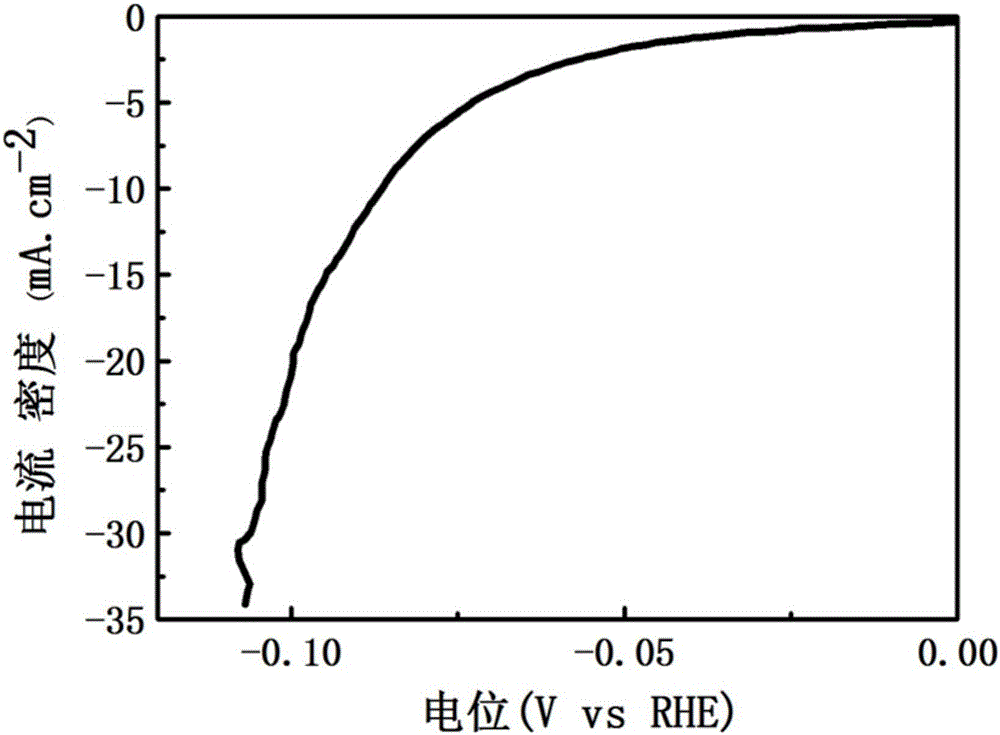
Preparation method of nickel phosphide hollow nano microspheres
A technology of nano-microspheres and nickel phosphide, applied in the preparation of microspheres, microcapsule preparations, etc., can solve the problems of high price, high specific surface area, and difficulty in putting into production, and achieve easy operation, simple process flow, and low cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Raw material NiCl 2 ·6H 2 O, NH 4 h 2 PO 4 and CH 3 The masses of COONa are 5g, 24.4g and 2.9g respectively, NiCl 2 ·6H 2 O, NH 4 h 2 PO 4 and CH 3 Dissolve COONa in 100ml deionized water, adjust the pH of the mixed solution to 8 with 1M KOH solution, put it in an oil bath at 90°C for 60min, filter and wash, freeze at -40°C for 24h and dry the obtained solid, and store in N 2 (Flow rate 100sccm) at 10°C / min to 300°C, keep warm for 60min, cool naturally, then soak in 20% HCl for 12h and freeze-dry.
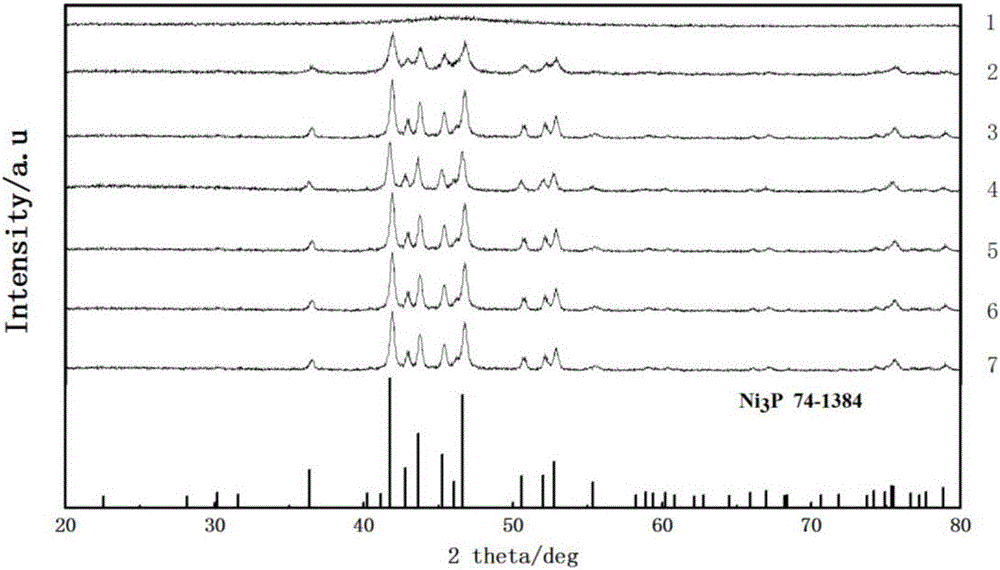
[0027] X-ray diffraction (XRD) showed that the obtained product was an amorphous phase.
Embodiment 2
[0029] The difference from Example 1 is:
[0030] Raw material NiCl 2 ·6H 2 O, NH 4 h 2 PO 4 and CH 3 The masses of COONa are 5g, 24.4g and 2.9g respectively, NiCl 2 ·6H 2 O, NH 4 h 2 PO 4 and CH 3 Dissolve COONa in 100ml deionized water, adjust the pH of the mixed solution to 8 with 1M KOH solution, put it in an oil bath at 90°C for 60min, filter and wash, freeze at -40°C for 24h and dry the obtained solid, and store in N 2 (Flow rate 100sccm) at 10°C / min to 400°C, keep warm for 60min, cool naturally, then soak in 20% HCl for 12h and freeze-dry.
[0031] X-ray diffraction (XRD) showed that the obtained product was pure Ni 3 P crystal phase.
Embodiment 3
[0033] The difference from Example 1 is:
[0034] Raw material NiCl 2 ·6H 2 O, NH 4 h 2 PO 4 and CH 3 The masses of COONa are 5g, 24.4g and 2.9g respectively, NiCl 2 ·6H 2 O, NH 4 h 2 PO 4 and CH 3 Dissolve COONa in 100ml deionized water, adjust the pH of the mixed solution to 8 with 1M KOH solution, put it in an oil bath at 90°C for 60min, filter and wash, freeze at -40°C for 24h and dry the obtained solid, and store in N 2 (flow rate 100sccm) at 10°C / min to 500°C, keep warm for 60min, cool naturally, then soak in 20% HCl for 12h and freeze-dry.
[0035] X-ray diffraction (XRD) showed that the obtained product was pure Ni 3 P crystal phase.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com