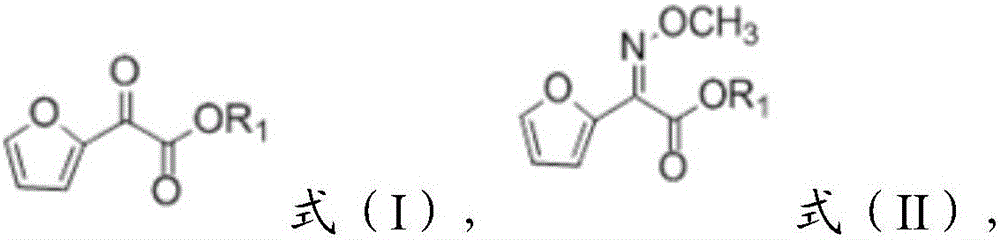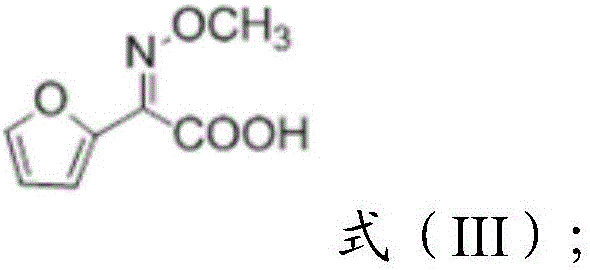Preparation method of 2-methoxyimino-2-furyl ammonium acetate
A technology of ammonium furyl acetate and methoxyimino, which is applied in the field of drug synthesis, can solve the problems of low total reaction yield, adverse effects on the occupational health of operators and the environment, and cumbersome processes, and achieve high yield and purity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
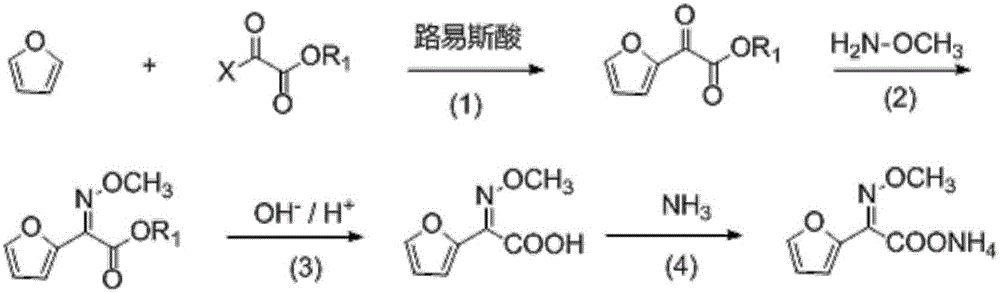
[0032] The invention provides a kind of preparation method of 2-methoxyimino-2-furyl acetate ammonium salt, comprising:
[0033] 1) reacting the compound of formula (I) structure with methoxyamine salt to obtain a reaction solution containing the compound of formula (II),
[0034]
[0035] where the R 1 It is C1~C8 alkyl group;
[0036] 2) adding alkaline hydrolysis to the reaction solution containing the compound of formula (II) obtained in step 1), to obtain a reaction solution containing the compound of formula (III),
[0037]
[0038] 3) adding ammonia to the reaction solution containing the compound of formula (III) to form a salt to obtain ammonium salt of 2-methoxyimino-2-furyl acetate.
[0039] According to the present invention, the present invention reacts the compound of formula (I) structure with methoxyamine salt to obtain the reaction solution containing the compound of formula (II); wherein, the compound of formula (I) structure can be isolated single T...
Embodiment 1
[0048] 1) Add 136g (2.00mol) of furan, 500g of dichloromethane and 136g (1.0mol) of zinc dichloride into the reaction vessel. The reaction temperature was controlled at 10°C, and 382 g (2.8 mol) of methyl chlorooxalate was added dropwise. After the addition was completed, the reaction was continued for 6h. After the reaction was complete, the reaction mixture was added to water. Stirring, extraction and separation, the obtained organic phase containing 2-oxofuryl acetate was directly used for the next reaction.
[0049] 2) Add 414 g (3.0 mol) of potassium carbonate to the organic phase containing 2-oxofuryl acetate obtained in step 1). The temperature of the system was kept at 0° C., and 184 g (2.2 mol) of methoxyamine hydrochloride was slowly added for not less than 1 hour. After the addition was completed, the reaction was continued at 0°C for 8h. HPLC monitoring of trans by-products was less than 5%. After the reaction is finished, the solution system containing the pr...
Embodiment 2
[0054] 1) Add 136g (2.00mol) furan, 500g dichloroethane and 80g (0.6mol) aluminum trichloride to the reaction vessel, control the reaction temperature at 10°C, add dropwise 300g (2.2mol) ethyl chlorooxalate ester. After the addition was completed, the reaction was continued for 6h. After the reaction was complete, the reaction mixture was added to water. Stir, extract and separate. The obtained organic phase containing 2-oxofuryl acetate was directly used for the next reaction.
[0055] 2) Add 328 g (4.0 mol) of sodium acetate to the organic phase containing 2-oxofuryl acetate obtained in step 1). The temperature of the system was kept at 0° C., and 250 g (3.0 mol) of methoxyamine hydrochloride was slowly added for not less than 1 hour. After the addition was completed, the reaction was continued at 0°C for 8h. HPLC monitoring of trans by-products was less than 5%. After the reaction is finished, the solution system obtained containing the product cis-2-methoxyimino-2-fu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com