Aspergillus oryzae lipase, encoding gene, vector, engineering bacterium and application thereof
A technology for encoding Aspergillus oryzae lipase and lipase, which can be used in genetic engineering, application, plant genetic improvement and other directions, and can solve the problems of high cost of enzyme extraction and purification, limited source of raw materials, and low activity of animal lipase.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
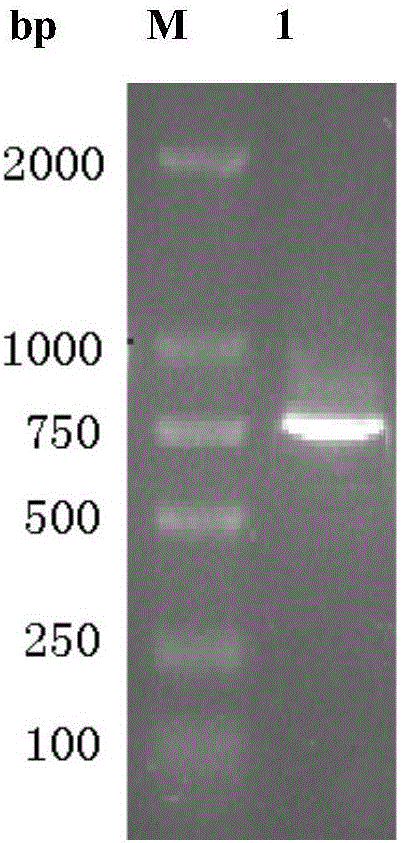
[0035] Starting from the original strain Aspergillus oryzae WZ007 cells, after a series of separation and purification work, the pure enzyme of Aspergillus oryzae WZ007 lipase was obtained, and its partial amino acid sequence was obtained by protein mass spectrometry analysis. PCR primers 1 and 2 were designed on the basis of the amino acid sequence information. Using a nucleic acid extraction kit (purchased from Takara Company) to extract the genomic DNA of Aspergillus oryzae (AspergillusoryzaeWZ007) WZ007, using the genomic DNA as a template, primer 1 (5'-ATGCATCTTGCTATCAAGTCTC-3'), primer 2 (5'-TTAGTTCGCAGCCGCAAC- 3') for PCR amplification.
[0036] The amount of each component in the PCR reaction system (total volume 100 μL):
[0037] 10×DNA Polymerase Buffer 10 μL, dNTP mixture 1 μL, primer 1 and primer 2 at a concentration of 50 μM 0.5 μL each, genomic DNA 1 μL, TransTaq DNA polymerase 2 μL, nucleic acid-free water 85 μL.
[0038] The PCR reaction conditions were as fol...
Embodiment 2
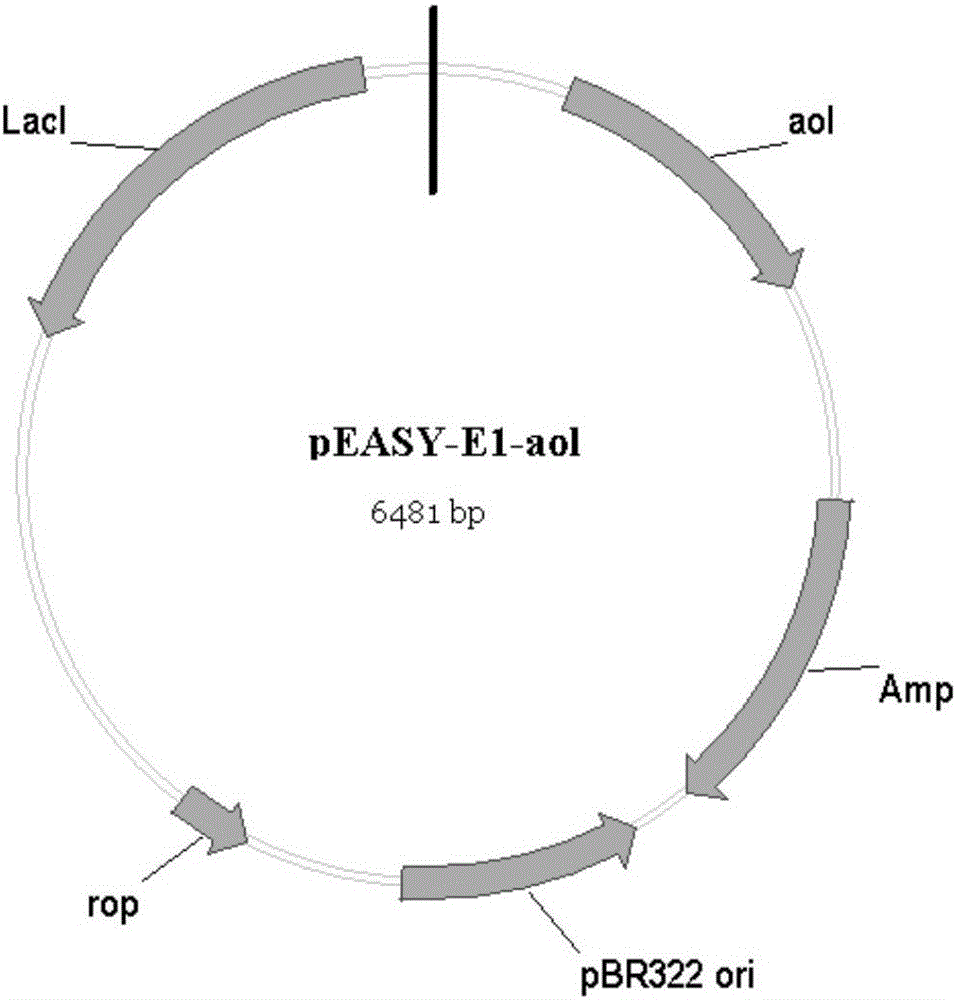
[0041] The recombinant plasmid obtained according to Example 1 was chemically transformed into Escherichia coli Trans B (DE3) (the competent cells were purchased from Beijing Quanshijin Biotechnology Co., Ltd.), and the recombinant plasmid pEASY-E1-aol containing intracellular expression was obtained. Recombinant Escherichia coli Trans B(DE3) / pEASY-E1-aol. The recombinant Escherichia coli was cultured at 30°C for 12 hours with LB liquid medium containing ampicillin (50 μg / ml), and then inoculated into fresh LB liquid containing ampicillin (50 μg / ml) at a 2% inoculum size (v / v). culture medium at 37°C to the cell concentration OD 600 About 0.4-0.6, then add IPTG with a final concentration of 0.2mM to the LB liquid medium, induce culture at 28°C for 8-10h, then centrifuge at 10000rpm at 4°C for 10min, and collect the bacterial cells containing recombinant lipase. A small amount of Tris-HCl buffer (50 mM, pH 8.0) was added, and vacuum freeze-dried at -60° C. for 2 days to obtain...
Embodiment 3
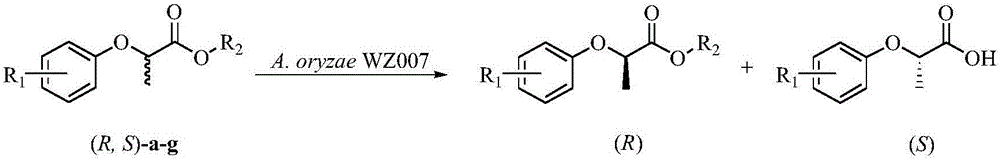
[0046] Using Escherichia coli Trans B(DE3) / pEASY-E1-aol freeze-dried cells containing recombinant lipase obtained in Example 2 as a biocatalyst, catalytic resolution of (R,S)-2-(4-hydroxyphenoxy ) ethyl propionate to prepare (R)-2-(4-hydroxyphenoxy) ethyl propionate. In a 5mL reaction system, take 50mg of lyophilized recombinant bacteria (10g / L), add 5mL of 0.2M phosphate buffer with pH 8.0, shake and mix well to make a bacterial suspension, add the substrate to eliminate Spin the EHPP (see Table 1), and carry out the hydrolysis reaction in a water-bath shaker at 30° C. and 250 rpm for 2 hours. After the reaction, adjust the pH to 2 with 4M dilute hydrochloric acid, then add 5 mL of ethyl acetate for extraction, take 20 μL of the organic layer, spin dry with a vacuum rotary evaporator, then add 1 mL of mobile phase to dissolve, remove water and make a liquid phase detection Samples were analyzed by liquid chromatography on the substrate and its conversion products. CHIRALPAK...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com