Eye drops
A technology of eye drops and Scutellaria baicalensis extract, applied in the field of medicine, can solve problems such as antibiotic abuse and drug resistance, and achieve the effect of less irritating effect and alleviating eye interference.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 78 parts by mass of normal saline, 26 parts of methylcellulose, 13 parts of sodium hyaluronate, 7 parts of taurine, 3 parts of triazole thioether indole derivatives , 14 parts [Ni(en) 3 ](L) 2 ·3H 2 O, 10 parts of hawthorn extract, 8 parts of Scutellaria baicalensis extract and 7 parts of preservative are uniformly mixed at normal temperature, wherein, the preservative consists of 8 parts of potassium sorbate by mass, 7 parts of ethyl paraben, 3 parts of sodium benzoate and Composed of 3 parts of sodium sulfite, after mixing evenly, the radiation intensity is 15kGy and the radiation dose rate is 3.6kGy / h. 60 Co-gamma radiation sterilization.
Embodiment 2
[0034] Dissolve 0.5mmol of nickel sulfate in 10mL of ethanol-water mixed solvent with a volume ratio of 1:l, and dissolve 1mmol of HL in 10mL of ethanol-water solvent, adjust the pH value of the solution to 5 with sodium hydroxide solution, It was gradually dropped into the above solution. After stirring at room temperature for 4 h, a large number of light green precipitates were formed, which were filtered, washed three times with 1:1 ethanol-water mixed solvent, and dried in vacuum at room temperature. Dissolve the obtained solid in 30 mL of ethanol under constant stirring, then add 1 mmol of ethylenediamine dropwise, the color of the solution changes from light green to lavender, stir at room temperature for 3 h, let the solution stand still, and obtain A purple single crystal was obtained, which was filtered, washed with a small amount of ethanol, and dried at room temperature, with a yield of 40% (calculated as nickel sulfate). [Ni(en) 3 ](L) 2 ·3H 2 O, IR (KBr) cm -...
Embodiment 3
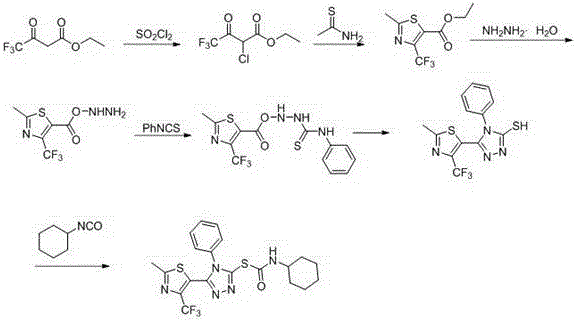
[0036] Triazole mercaptoformamide derivatives are synthesized via the following route,
[0037] The synthesis methods of compound 2 and compound 3 are prior art.
[0038] Synthesis of Compound 4: Add 5.0 g (21.0 mmol) of Compound 3 and 3.0 mL (63.0 mmol) of 85% hydrazine hydrate (molar ratio 1:3) into a 50 mL round bottom flask, and let it stand for 48 h. After the reaction was complete, it was filtered with suction, and the filter cake was washed with water and dichloromethane in turn, and dried in vacuo to obtain a light yellow solid (compound 4).
[0039] Compound 5: Add 2.3 g (10.0 mmol) of compound 4 and 40.0 mL of absolute ethanol into a 100 mL three-necked flask, and drop 10 mmol of phenyl isothiocyanate after the solid is completely dissolved, reflux for 3 hours, cool, and a large amount of The white solid compound 5 was suction filtered, washed with a small amount of deionized water, and dried to obtain a white solid.
[0040] Synthesis of compound 6: 3.6 g (10.0 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com