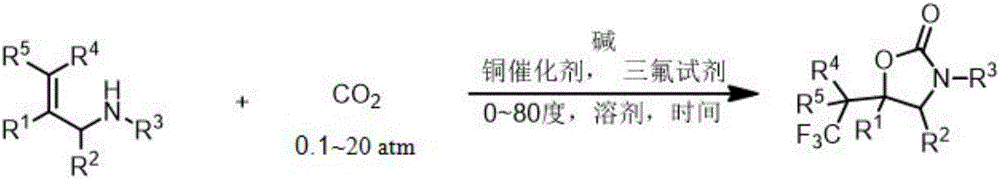
Fluorine-containing heterocyclic compound and preparation method thereof
A compound and substrate technology, applied in the field of preparation of 2-oxazolinone compounds, to achieve the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
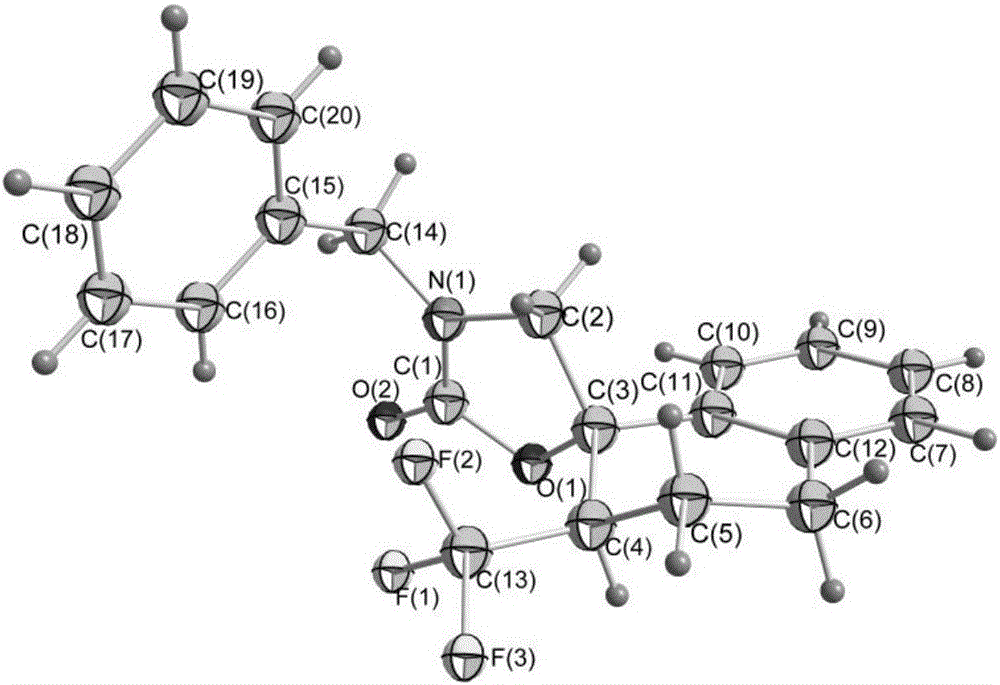
Image
Examples
Embodiment 1
[0088] Example 1 3-benzyl-5-phenyl-5-(2,2,2-trifluoroethyl)-2-oxazolinone (3-benzyl-5-phenyl-5-(2,2, 2-trifluoroethyl) oxazolidin-2-one) (hereinafter referred to as compound 2a) preparation
[0089]
[0090] Table 1: Optimal selection of reaction conditions
[0091]
[0092]
[0093] [a]: The yield listed in the table is the GC yield using n-dodecane as the internal standard, and the separation yield is in brackets; [b]: the amount of alkali is 0.6mmol; [c]: 25 degrees Celsius; [d ]: use the first generation of Togni reagent; [e]: use Umemoto reagent; N.D. = not detected; [f]: 80 degrees Celsius; [g] 0.1atm; [h] 20atm.
[0094] According to the conditions shown in Table 1, compound 2a was prepared with 1a as the substrate. The specific operation was as follows: 0.04mmol catalyst and 0.44mmol second-generation Tognis reagent (139mg) were added to a 25mL Schlenk tube with a magnet, and the above operations were carried out in a glove box. completed, then take the reac...
Embodiment 2 to Embodiment 32
[0097] Example 2 to Example 32 Preparation of 2-oxazolinone compounds 2aa, 2ab, 2ac, 2ad, 2ae, 2af, 2b-2z
[0098] Explanation: 2-oxazolone compound 2aa means that the compound is prepared from substrate 1aa, and the naming rules of other 2-oxazolone compounds are the same.
[0099] Prepare 2-oxazolinone compounds 2aa, 2ab, 2ac, 2ad, 2ae, 2af, 2b-2z according to the conditions shown in Table 2 and Table 3. The specific operations are as follows: add 0.04mmol tetraethylcyanide copper hexafluorophosphate (14.9mg), 0.44mmol second-generation Tognis reagent (139mg), 0.40mmol allylamine derivative (if the substrate is solid), the above operations are completed in the glove box, and then The reaction tube was taken out of the glove box, and the 2 Gas replacement three times, followed by CO 2 Add 0.40mmol allylamine derivative (if the substrate is a liquid), 4mL anhydrous acetonitrile, 0.80mmol (120μL) 1,8-diazabicyclo[5.4.0]undec-7- ene (DBU). Then, at one atmosphere of CO 2 Ti...
Embodiment 33 to Embodiment 35
[0250] Application of Example 33 to Example 35 Compound 2a
[0251]
[0252] Compounds 3, 4 and 5 were obtained in high yields from compound 2a. The specific preparation method is as follows.
[0253] N-Benzyl-N-(4,4,4,-trifluoro-2-hydroxy-2-phenylbutyl)-3-enbutyramide (compound 3)
[0254] N-benzyl-N-(4,4,4-trifluoro-2-hydroxy-2-phenylbutyl)but-3-enamide
[0255]
[0256] To a solution of 0.2 mmol (67 mg, 1.0 equiv) of compound 2a in THF (1 mL) was added dropwise 0.6 mmol (3.0 equiv) of allylmagnesium bromide at -78°C. After maintaining the reaction at the above temperature for 3 hours, add 1 mL of saturated ammonium chloride solution, and when the reaction system rises to room temperature, extract 3 times with diethyl ether (3×5 mL), then spin dry, and perform column chromatography (PE:EA= 5:1) separation and purification to obtain the target compound 3 (72 mg) as a colorless oily liquid with a yield of 96%.
[0257] R f (pentane / EtOAc 5:1) = 0.5; 1 H NMR (400MHz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com