Molecularly targeted aza aromatic ring axially substituted phthalocyanine complex and preparation method thereof
A heteroaromatic ring, molecular targeting technology, applied in medical preparations containing active ingredients, pharmaceutical formulations, silicon organic compounds, etc., can solve the problems of poor selectivity, high toxicity, etc. Beneficial to industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
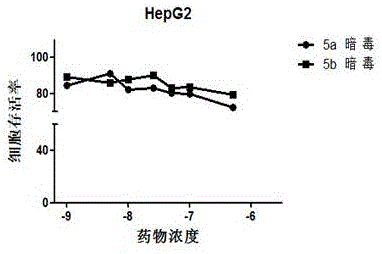
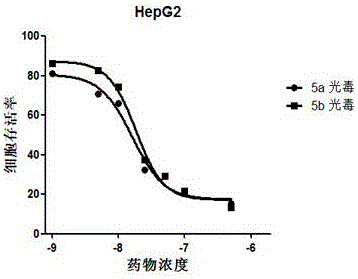
Image
Examples
Embodiment 1
[0027] Example 1 (M=Si, n=4)
[0028] (1) Add 23.29 g (0.12 mol) of tetraethylene glycol and 15.18 g (0.15 mol) of triethylamine in sequence into a 250 mL round bottom flask equipped with a magnetic stirring device, an air duct device and a reflux condensing device and 50 mL of dichloromethane, then dissolve 5.72 g (0.03 mol) of p-toluenesulfonyl chloride in 100 mL of dichloromethane, and install it in a constant pressure dropping funnel, slowly drop the p-toluenesulfonyl chloride solution under nitrogen protection Added to the reaction flask, then stirred at room temperature for 8 h. After the reaction was finished, extract three times with 1 mol / L hydrochloric acid solution and saturated sodium chloride solution respectively, collect the organic layer, and then remove dichloromethane by distillation under reduced pressure; The developer is a mixed solution of petroleum ether: ethyl acetate = 1:1, and about 8.35 g of light yellow viscous liquid (ie compound 2a) is obtained, ...
Embodiment 2
[0032] Example 2 (M=Si, n=5)
[0033] (1) Add 10.76 g (0.12 mol) pentaethylene glycol, 4.57 g (0.15 mol) triethylamine and 50 mL Dichloromethane, then dissolve 2.15g (0.03 mol) p-toluenesulfonyl chloride in 100 mL of dichloromethane, and install it in a constant pressure dropping funnel, and slowly drop the p-toluenesulfonyl chloride solution to the reaction under nitrogen protection bottle, and then stirred at room temperature for 10 h. After the reaction was finished, extract three times with 1 mol / L hydrochloric acid solution and saturated sodium chloride solution respectively, collect the organic layer, and then remove dichloromethane by distillation under reduced pressure; The developer is a mixed solution of petroleum ether: ethyl acetate = 1:1, and about 6.15 g of a light yellow viscous liquid (compound 2b) is obtained, with a yield of 83%;
[0034] (2) Add 1.01 g (4.50 mmol) N-(3-chloro-4-fluorophenyl)-7-methoxy to a 100 mL round bottom flask equipped with a magnetic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com