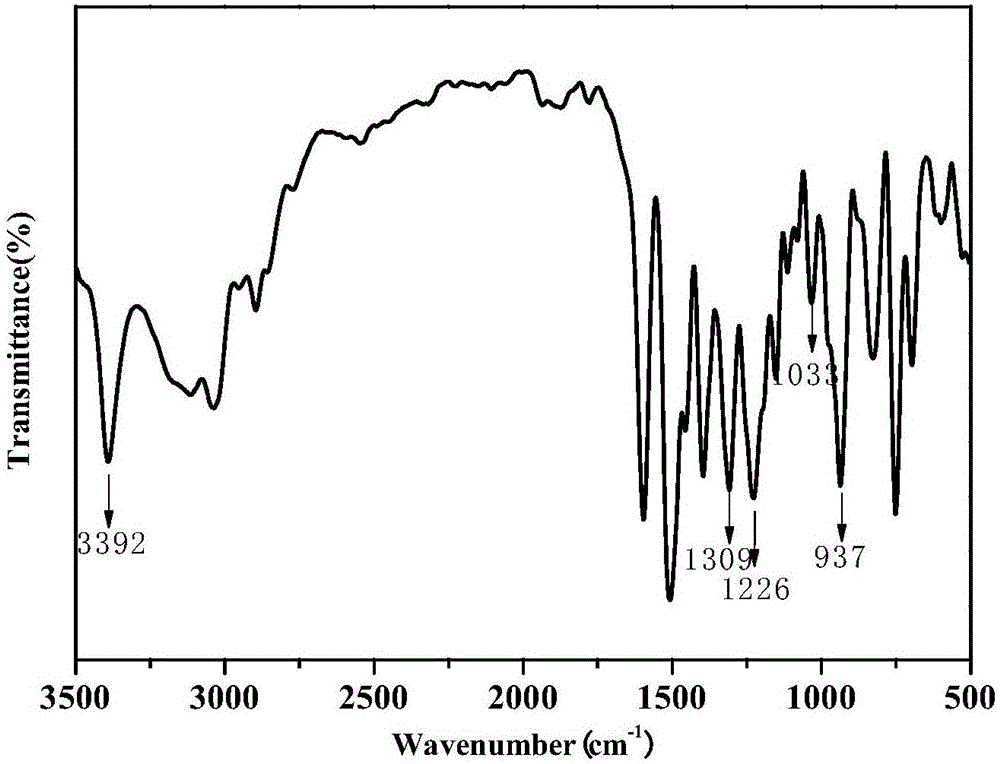
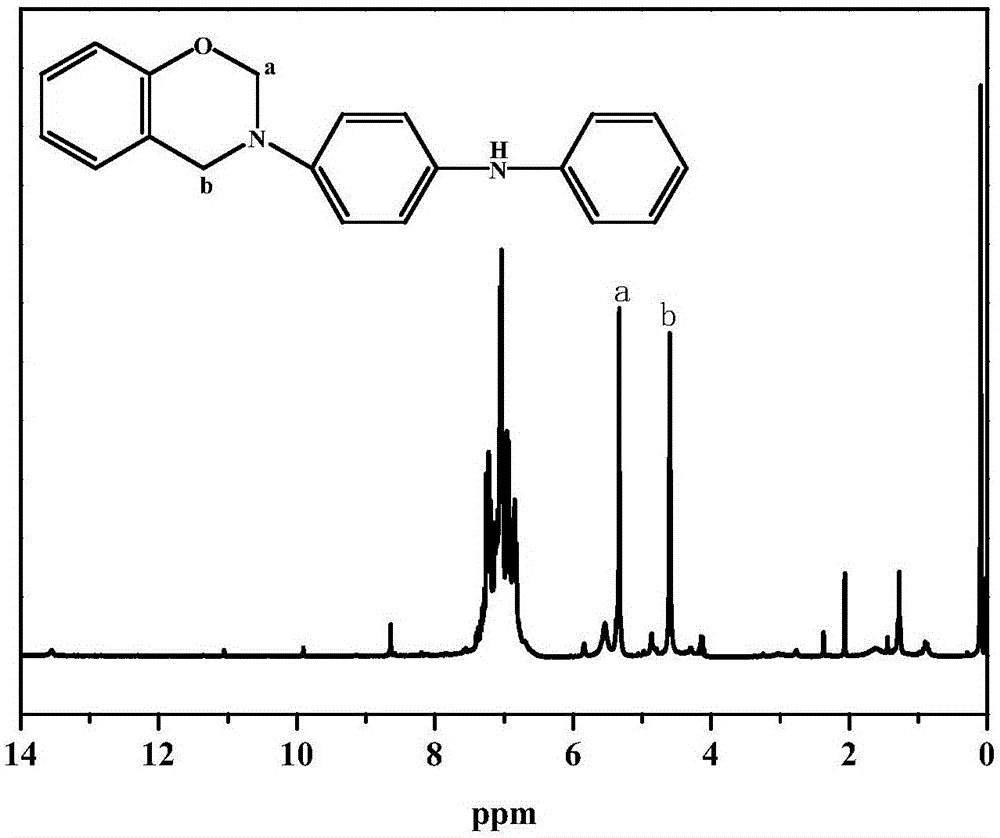
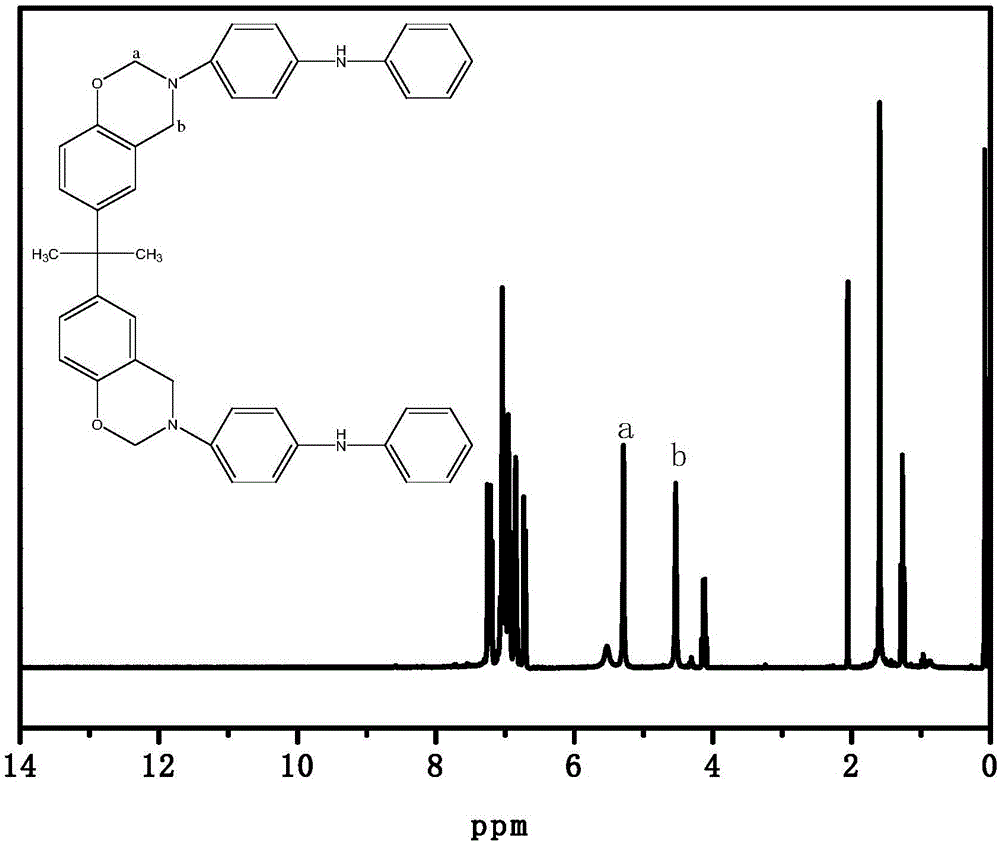
Benzoxazine resin with electroactivity and preparation method thereof
A benzoxazine and electroactive technology, which is applied in the field of new electroactive benzoxazine resin and its preparation, can solve the problem that the performance cannot meet the needs of the electronic field, the application research of benzoxazine is less, and there is no polymer, etc. problems, to achieve the effect of easy industrial production, low softening temperature and simple curing process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0071] A kind of preparation method of making described electroactive benzoxazine provided by the present invention, the steps are as follows:
[0072] 1. After adding 35-70 parts by weight of paraformaldehyde and 70-305 parts by weight of phenol into the reaction kettle, add 600-1000 parts by weight of dispersion medium, and mechanically stir for 1-3 hours at 0-30 ° C. The stirring speed is 300-600 rpm, then raise the temperature of the system to 40-50°C;
[0073] 2. Disperse 170-440 parts by weight of the electroactive primary amine intermediate into 200-600 parts by weight of the dispersion medium, and gradually add it to the system described in step S1.2. After the electroactive primary amine intermediate is added, the system is reacted at 40-60°C for 6-12 hours in a nitrogen atmosphere and protected from light;
[0074] 3. After step 2 is completed, the temperature of the reaction solution is raised to 65-150°C for reflux reaction for 1-3 hours;
[0075] 4. After step 3...
Embodiment 1
[0078] 70 parts by weight of paraformaldehyde, 185 parts by weight of phenol and 600 parts by weight of toluene were added to the reaction kettle, mechanically stirred at 25°C for 1 hour at a stirring speed of 400 rpm, and then the temperature of the system was raised to 40°C. Disperse 170 parts by weight of the electroactive primary amine intermediate of structure I in 200 parts by weight of toluene, and gradually and slowly add it to the mixed solution of paraformaldehyde and phenol. After the electroactive primary amine intermediate of structure type I was added, the temperature of the system was raised to 50°C, and after reacting for 7 hours in a nitrogen atmosphere and protected from light, the temperature was rapidly raised to 120°C, and the reaction was refluxed for 2 hours. After the reaction was completed, the reaction was stopped and cooled to room temperature. After the reaction solution was washed with alkali and water three times each, the organic phase was separa...
Embodiment 2
[0085] 70 parts by weight of paraformaldehyde, 230 parts by weight of bisphenol A and 600 parts by weight of toluene were added into the reaction kettle, mechanically stirred at 600 rpm for 2 hours at 30°C, and then the temperature of the system was raised to 50°C. Disperse 350 parts by weight of structure I electroactive primary amine intermediate in 600 parts by weight of toluene, and gradually add it into the mixed solution of paraformaldehyde and bisphenol A gradually. After the electroactive primary amine intermediate of structure type I was added, the temperature of the system was raised to 60°C, and after reacting for 8 hours in a nitrogen atmosphere and protected from light, the temperature was rapidly raised to 125°C, and the reaction was refluxed for 3 hours. After the reaction was completed, the reaction was stopped and cooled to room temperature. After the reaction solution was washed with alkali and water four times each, the organic phase was separated. The tolu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com