Preparing method and application of cell immunologic adjuvant TSA-41
A technology of TSA-41 and cellular immunity, applied in the field of biomedicine, can solve problems such as nucleic acid safety hazards, and achieve the effects of being beneficial to industrial production, improving preparation efficiency, and avoiding process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
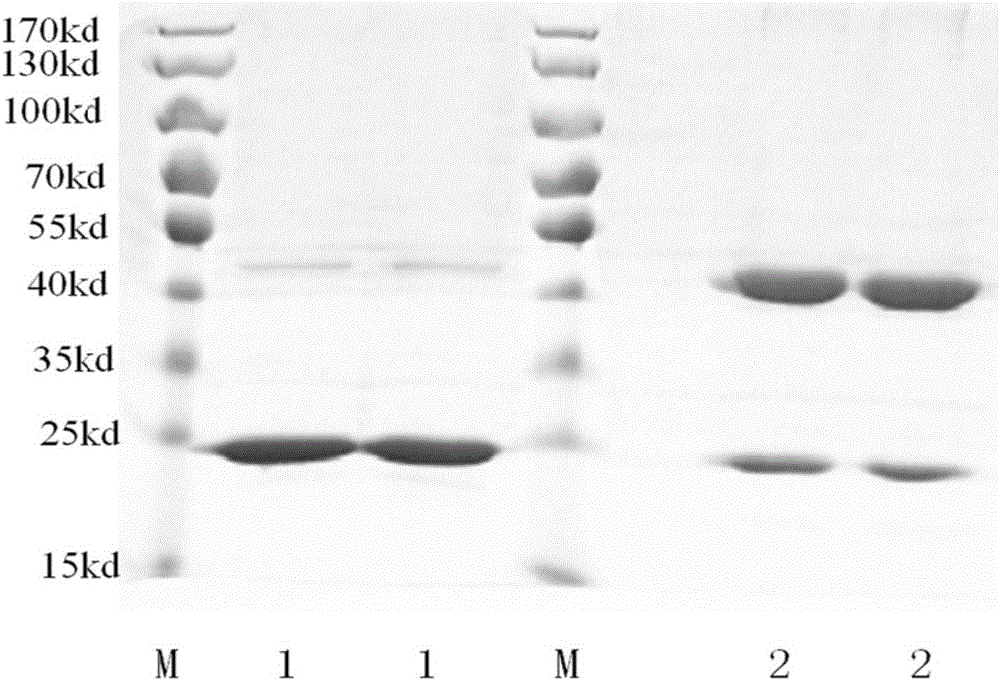
[0038] The preparation of embodiment 1 TSA-41 adjuvant
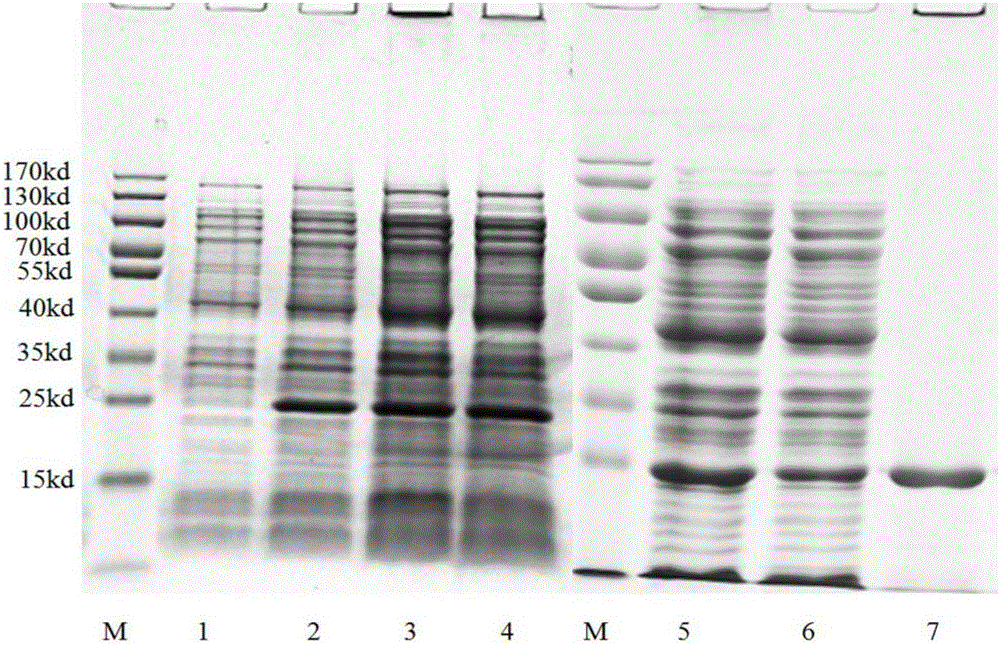
[0039] (1) Synthesize the gene sequence of SEQ ID NO.1 by artificial gene synthesis technology, and insert the gene of SEQ ID NO.1 into pET28a Escherichia coli expression plasmid through the two restriction sites of NdeI and XbaI to obtain the expression of the gene of SEQ ID NO.1 The sequence of recombinant plasmid SEQ ID NO.1-pET28a.
[0040](2) Take a 1.5 ml centrifuge tube containing 50 microliters of BL21 (DE3) Escherichia coli competent cells and place it on ice for 5 minutes, add 50 nanograms of SEQ ID NO.1-pET28a recombinant plasmid, mix gently, And incubate on ice for 30 minutes; place the centrifuge tube in a 42°C water bath for 90 seconds, quickly take it out and place it on ice for 5 minutes; add 900 microliters of LB liquid medium to the centrifuge tube, and culture with shaking at 37°C for 45 Minutes, take 200 microliters of the culture product and spread it on an LB solid medium plate with a diameter of 1...
Embodiment 2
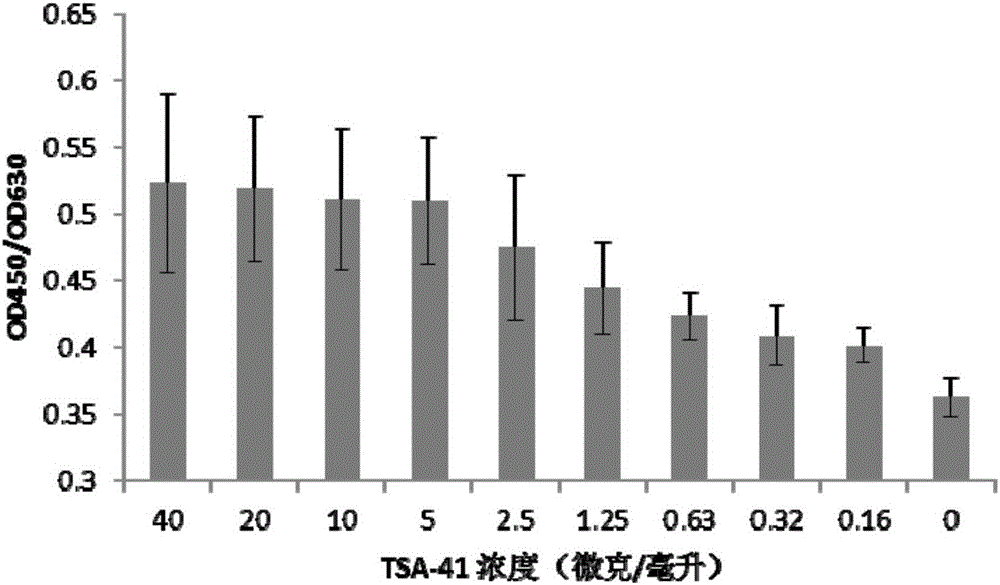
[0054] Example 2 Effect of TSA-41 adjuvant on proliferation of mouse lymphocytes in vitro
[0055] (1) The C57 / BL mice were sacrificed, and the spleen was taken and crushed gently under sterile conditions to obtain a spleen cell suspension.
[0056] (2) The obtained spleen cell suspension was treated with erythrocyte lysate for 5 minutes, centrifuged at 1000 rpm to discard the supernatant, then washed twice with serum-free RPMI-1640 medium, and finally the cell pellet was resuspended in a certain amount of serum RPMI In -1640 medium, the mouse spleen lymphocyte suspension was obtained.
[0057] (3) Dilute the mouse spleen lymphocyte suspension to 5×10 6 cells / ml, seeded in 96-well cell culture plate, 100 μl per well.
[0058] (4) Dilute the TSA-41 adjuvant with cell culture medium to a series concentration of 4 mg / ml, 2 mg / ml, 1 mg / ml, 0.5 mg / ml, 0.25 mg / ml, 0.125 mg / ml, 0.063 mg / ml ml, 0.032 mg / ml, and 0.016 mg / ml, take 1 microliter of adjuvant of each concentration and ad...
Embodiment 3
[0062] Example 3 Enzyme-linked dot immunoassay detection of TSA-41 adjuvant's immune enhancement effect on human papillomavirus recombinant protein vaccine for treatment
[0063] (1) There were three C57 / BL mice in each group of experimental animals, which were divided into three groups: TSA-41 adjuvant group, human papillomavirus recombinant protein vaccine group for treatment, human papillomavirus recombinant protein vaccine for treatment and TSA- 41 adjuvant co-immunization groups. Among them, the inoculation volume of human papillomavirus recombinant protein vaccine for treatment was 100 micrograms, and the inoculation volume of TSA-41 adjuvant was 5 micrograms. Each mouse was boosted immunized two days apart, and inoculated three times in total.
[0064] (2) The mice were sacrificed on the 10th day of the initial immunization, and the spleen was removed and crushed gently under aseptic conditions to obtain a spleen cell suspension.
[0065] (3) The obtained spleen cell s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com