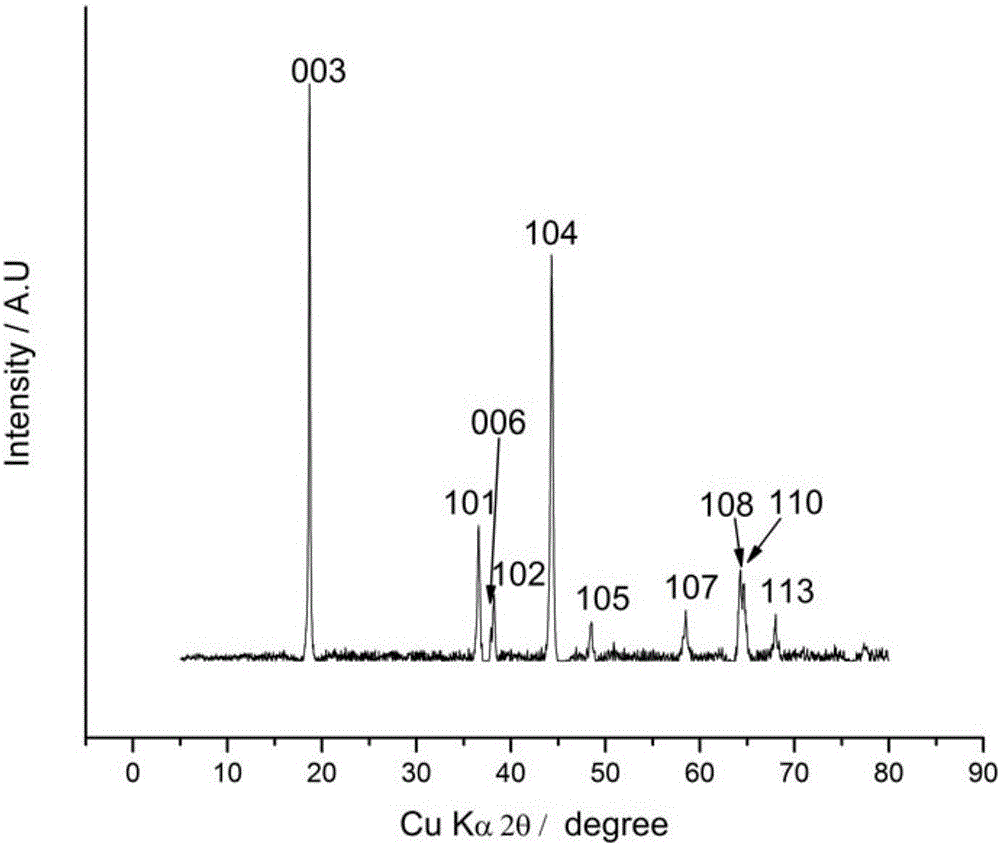
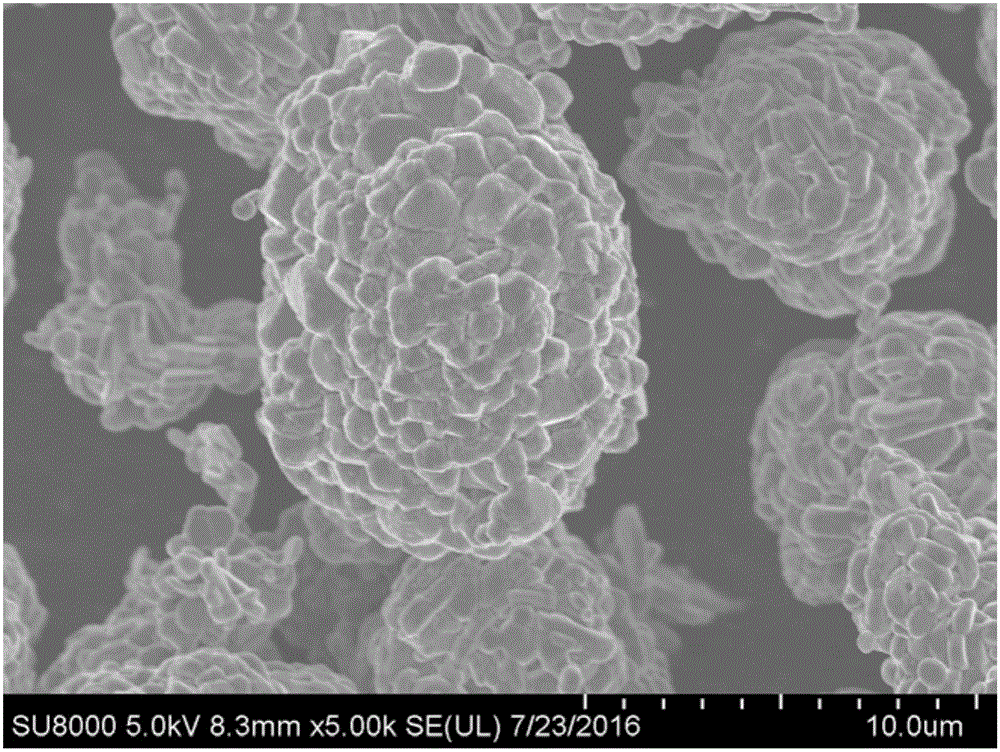
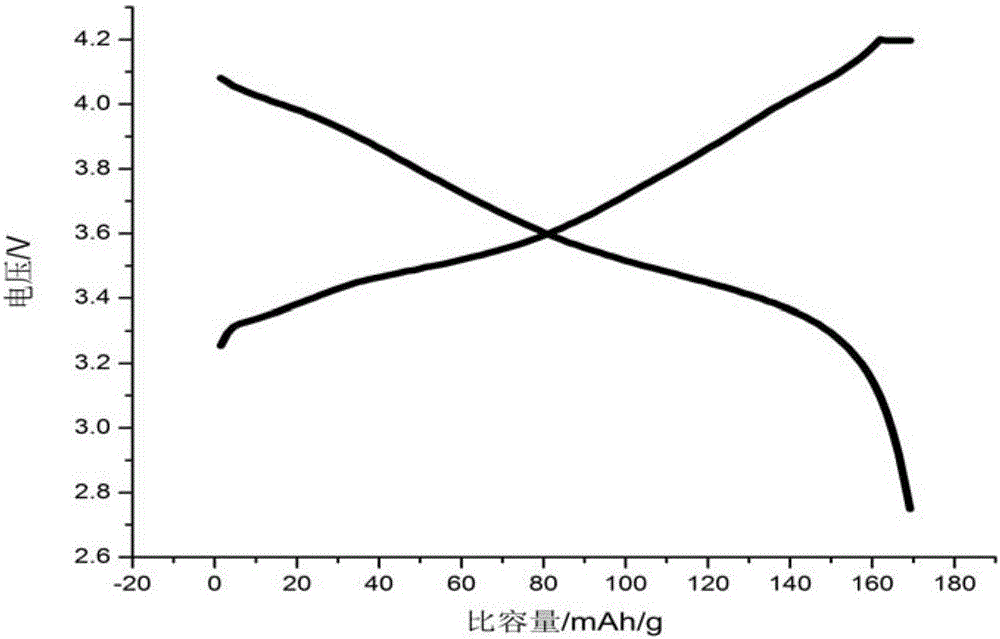
BN-coated cobalt-free Ni-Mn solid solution Ni-based positive electrode material
A cathode material and coating technology, which is applied in the field of BN-coated cobalt-free Ni-Mn solid-solution nickel-based cathode materials, can solve the problems of high preparation cost, difficulty in large-scale production, and poor uniformity of ion distribution, so as to improve cycle performance , strong acid resistance, and the effect of stabilizing the material structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0037] A BN-coated cobalt-free Ni-Mn solid-solution nickel-based positive electrode material, the structural formula of which is: LiNi x mn 1-x o 2 ·a BN, where 0.5≤ x x mn 1-x o 2 A coating on the surface of a material.
[0038] The preparation method comprises the following steps:
[0039] (1) Weigh the nickel salt and the manganese salt according to the ratio, dissolve the nickel salt and the manganese salt in deionized water, prepare a transition metal source solution, and adjust the pH of the solution to 7~7.2 with ammonia water; the nickel salt is selected from chlorine One or more of nickel oxide, nickel sulfate, nickel nitrate, nickel acetate. The manganese salt is selected from one or more of manganese chloride, manganese sulfate, manganese nitrate and manganese acetate.
[0040] (2) Precipitant plus deionized water is prepared into a primary solution with a system of 0.1-10moL / L, and a complexing agent is added to the primary to mix to obtain a precipitant sol...
Embodiment 1
[0049] NiSO 4 , MnSO 4 Dissolve in deionized water at a molar ratio of 0.8:0.2 to make 2mol L -1 Transition metal source solution, the precipitant NaOH was dissolved in deionized water to prepare 4mol L -1 NaOH solution, add ammonia water accounting for 5% of the volume of the sodium hydroxide solution to the NaOH solution, pump the transition metal source solution and the sodium hydroxide solution into the reactor at a flow rate of 50mL / h at the same time, and control the temperature of the reactor to 55 ℃, the reaction pH value is 11.3, carry out co-precipitation reaction, keep stirring at the reaction temperature for 3 hours after the reaction is completed, then let it stand for 8 hours, wash the precipitate with deionized water while suction filtering until the precipitated impurity content is less than 400ppm, and obtain after drying Precursor Ni 0.8 mn 0.2 (OH) 2 .
[0050] Precursor and lithium hydroxide are according to the ratio of the molar number of lithium t...
Embodiment 2
[0055] LiNi 0.8 mn 0.2 o 2 The preparation process of the material is the same as in Example 1.
[0056] 50 g of cathode material LiNi 0.8 mn 0.2 o 2 Dissolve in ethanol solution of diboron trioxide, wherein the quality of diboron trioxide is 0.54g, ethanol is 800ml, at 800 rad min -1 The stirring rate was set at 70 °C, and the solution was evaporated to dryness within 5 h to obtain a modified nickel-based cathode material coated with boron trioxide.
[0057] The modified nickel-based cathode material coated with diboron trioxide was placed in a tube atmosphere furnace at 5°C min -1 The heating rate rises to 600-850°C at a flow rate of 500cm 3 min -1 Under nitrogen protection conditions, with a flow rate of 200 cm 3 min -1 Ammonia reacts, the reaction time is 4h, and the temperature is lowered to room temperature, and LiNi with a BN coating amount of 1%wt is obtained 0.8 mn 0.2 o 2 0.018BN.
[0058] Will LiNi 0.8 mn 0.2 o 2 0.009BN cathode material assembled...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com