Method for expressing and purifying soluble dermatophagoides pteronyssinus major allergen Der p 2 protein
A major allergen, soluble technology, applied in the biological field, can solve problems such as difficult to achieve standardization, false positives, stability problems, etc., and achieve the effect of high purity of the final product, low production cost, and easy purification.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
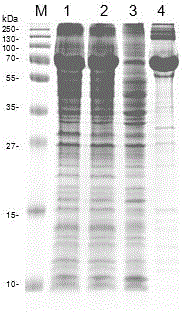
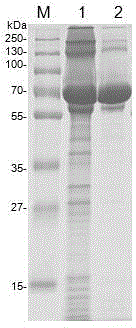
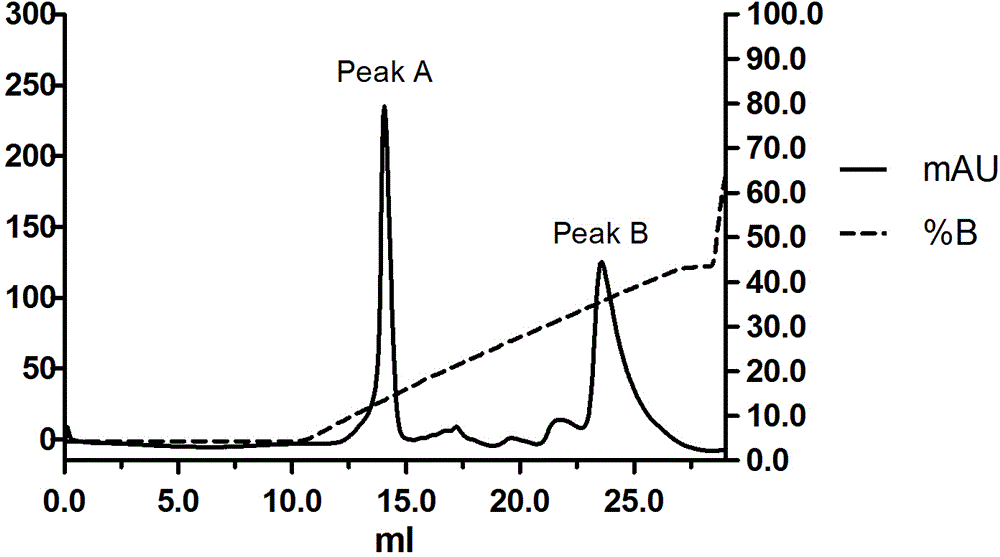
Image
Examples
Embodiment Construction
[0038] The present invention is further explained below in conjunction with the examples, but the examples do not limit the present invention in any form.
[0039] 1. Materials used
[0040] 1.1 The main reagents DNA restriction endonuclease BamHI, EcoRI, Phusion ultra-fidelity DNA polymerase and T4 DNA ligase are all products of NEB company; HRV-3C protease was expressed and purified by our research group; plasmid mini-extraction kit And gel recovery kit: product of Dalian Bao Biology Co., Ltd.; nickel agarose gel (Ni Sepharose TM 6 Fast Flow): product of General Electric Company of the United States; natural Der p 2: product of Indoor Biotech Company of the United Kingdom; biotin-labeled anti-human IgE Fc segment monoclonal antibody: product of Abcam Company of the United States; HRP-labeled streptavidin: product of the United States Products of Southern Biotech; bovine serum albumin (BSA): products of Sigma; nitrocellulose membranes: products of Pall Corporation of the Un...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com