Diimine ligand compound, and complex and application thereof
A technology of diimine ligands and complexes, which is applied in the preparation of imino compounds, nickel organic compounds, organic chemistry, etc., and can solve the problems that late transition metal catalysts cannot meet the requirements.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
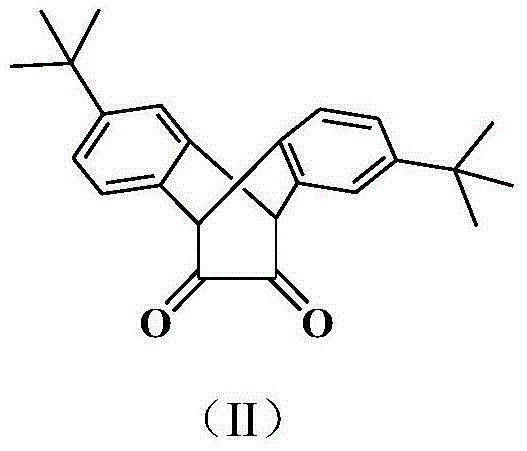
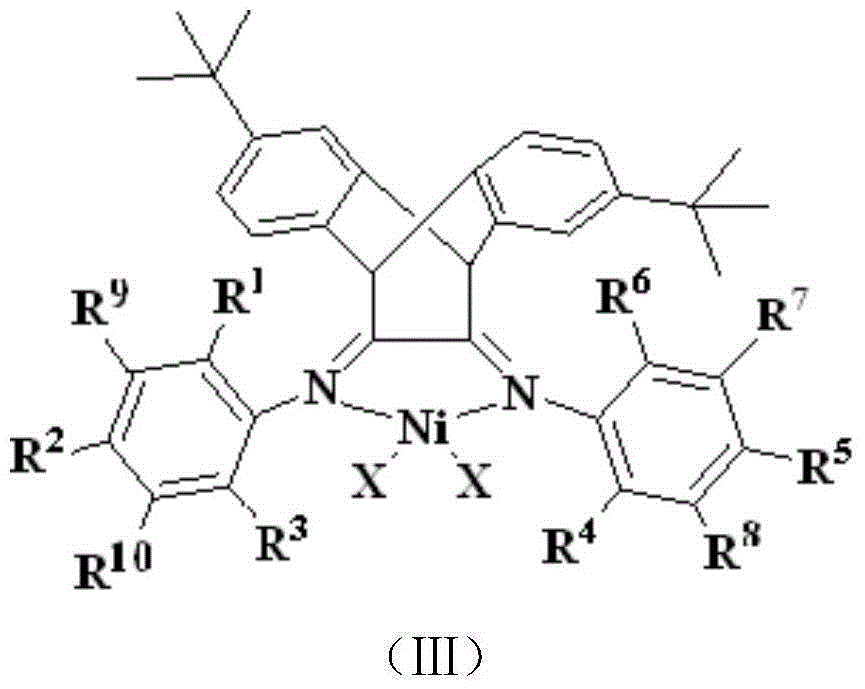
[0057] 1) preparation of ligand (R in structural formula (I) 1 , R 3 , R 4 and R 6 is methyl, R 2 and R 5 for hydrogen, R 7 -R 10 are hydrogen):
[0058] Compound A (1.66g, 4.8mmol) and 2,6-methylaniline (1.3ml, 10.4mmol), p-toluenesulfonic acid as a catalyst, refluxed in 100mL toluene for 1 day, removed the solvent after filtration, and the residue was treated with dichloro Methane was dissolved, and the column of perbasic alumina was rinsed with petroleum ether / ethyl acetate (20:1). The second fraction was divided into the product, and the solvent was removed to obtain a yellow solid with a yield of 85%. 1 H NMR (CDCl 3 , δ, ppm): 1.19 ppm (s, 18H), 1.88 (s, 12H), 4.70 (s, 2H), 7.04 (m, 10H), 7.12 (s, 2H).
[0059] 2) Preparation of complex 1:
[0060] 10ml (DME)NiBr 2 (277mg, 0.9mmol) in dichloromethane solution was added dropwise to 10ml of the above-mentioned ligand (498mg, 0.9mmol) in dichloromethane solution, stirred at room temperature for 6 hours, precipita...
Embodiment 2
[0064] 10atm ethylene polymerization: Dry the 1L stainless steel polymerization kettle equipped with mechanical stirring at 130°C for 6Hrs continuously, vacuumize while it is hot and use N 2Air replacement 3 times. Add 10.7 mg (10 μmol) of complex 1 prepared in Example 1 and then evacuate and replace with ethylene three times. 500 ml of hexane was injected, and 6.5 ml of methylaluminoxane (MAO) (1.53 mol / l toluene solution) was added to make Al / Ni=1000. At 100°C, the ethylene pressure was maintained at 10 atm, and the reaction was stirred for 30 min. Neutralize with ethanol solution acidified with 5% hydrochloric acid to obtain polyethylene with a polymerization activity of 1.44×10 6 g·mol -1 (Ni)·h -1 , and the results are shown in Table 1.
Embodiment 3
[0066] 1) preparation of ligand (R in structural formula (I) 1 , R 3 , R 4 and R 6 is ethyl, R 2 and R 5 for hydrogen, R 7 -R 10 are hydrogen):
[0067] Compound A (2.7g, 7.8mmol) and 2,6-diethylaniline (3.0ml, 17.4mmol), p-toluenesulfonic acid as a catalyst, refluxed in 100mL toluene for 1 day, removed the solvent after filtration, and the residue was washed with di Methane chloride was dissolved, and the column of perbasic alumina was rinsed with petroleum ether / ethyl acetate (20:1). The second fraction was divided into the product, and the solvent was removed to obtain a yellow solid with a yield of 81%. 1 H NMR (CDCl 3 , δ, ppm): 1.06(t, 12H, J=7.0Hz), 1.19ppm(s, 18H), 2.20(dd, 8H, J=7.0Hz), 4.70(s, 2H), 7.04(m, 10H ), 7.13(s, 2H).
[0068] 2) Preparation of complex 2: 10ml (DME) NiBr 2 (155mg, 0.5mmol) in dichloromethane solution was added dropwise to 10ml of the above-mentioned ligand (304mg, 0.5mmol) in dichloromethane solution, stirred at room temperature fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com