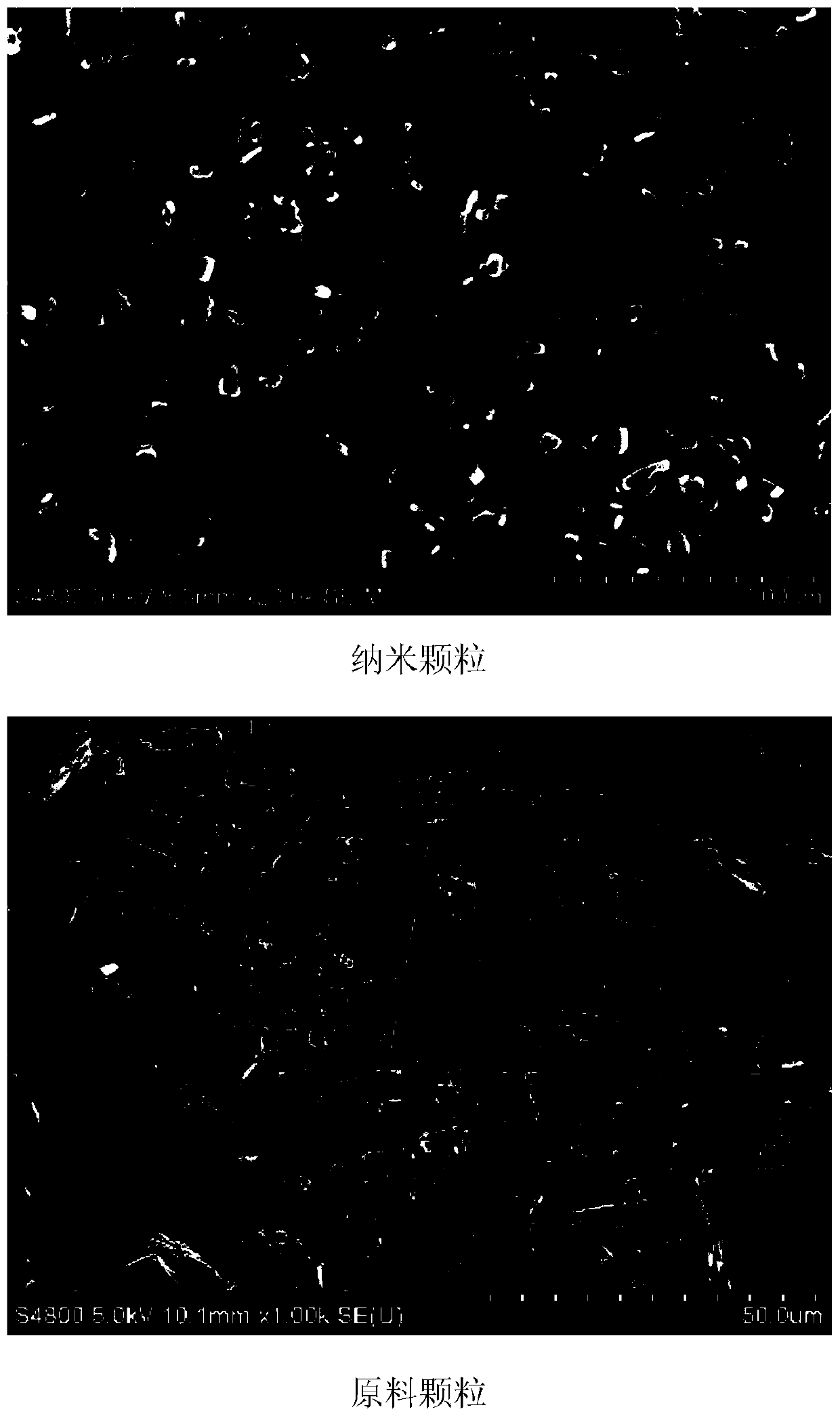
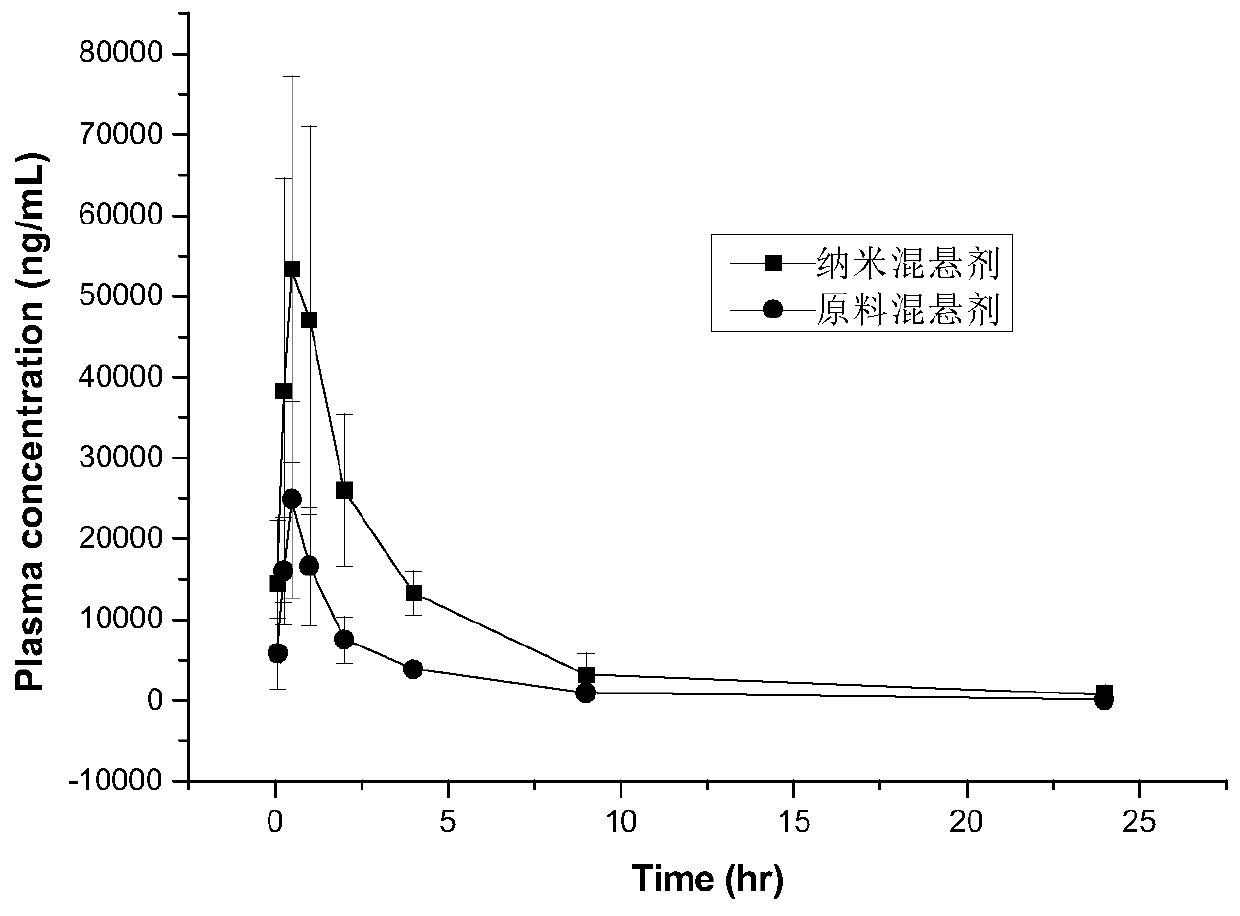
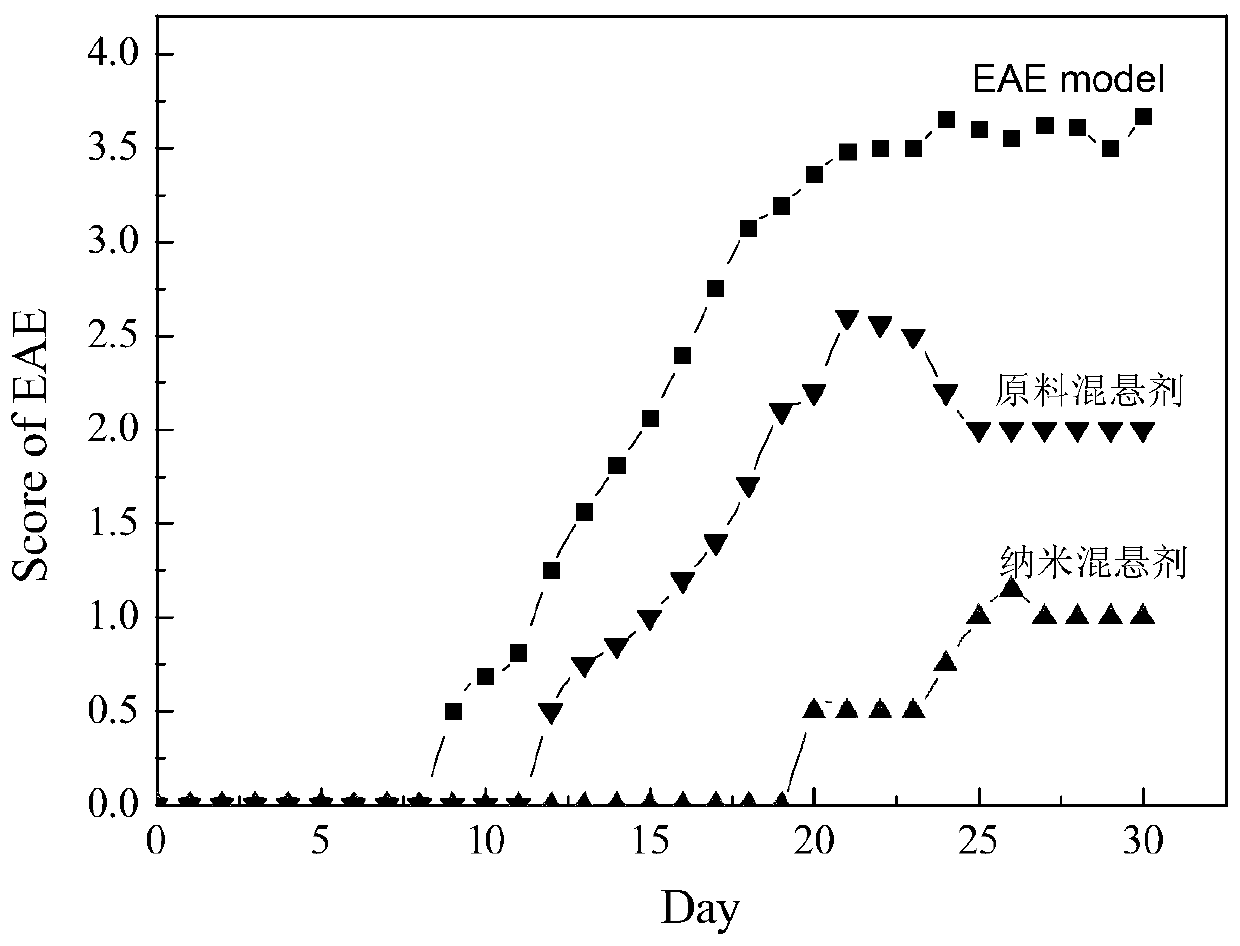
A kind of sirolimus nano-suspension and preparation method thereof
A nano-suspension, sirolimus technology, which is applied in pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., can solve the problems of high equipment requirements, easy metal residues, and high costs , to achieve the effect of good dispersion stability, low ethanol content and uniform particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Embodiment 1: Preparation of low concentration sirolimus nanosuspension (0.5mg / mL)
[0080]
[0081] Dissolve the sirolimus bulk drug in 40 mL of ethanol at room temperature, and filter through a 0.22 μm filter membrane to obtain a sirolimus solution. Then dissolve polyethylene glycol 4000, Tween 80, methylcellulose, sucrose and sodium benzoate in 2000 mL of water at room temperature, add water to make up to 2624 mL, and filter through a 0.22 μm filter membrane to obtain an aqueous solution of excipients. Finally, the sirolimus solution was poured into the auxiliary material aqueous solution, and the stirring paddle was stirred at 500 rpm for one minute to obtain the sirolimus nanosuspension. The average particle size is 249nm as tested by a nanometer particle size analyzer.
Embodiment 2
[0082] Embodiment 2: the preparation of high concentration sirolimus nanosuspension (3mg / mL)
[0083]
[0084] Sirolimus raw material and methylparaben were dissolved in 80 mL of ethanol at room temperature, and filtered through a 0.22 μm filter membrane to obtain a sirolimus solution. Then, dissolve povidone K30, sodium alginate, hydroxypropyl methylcellulose and sorbitol in 2500 mL of water at room temperature, add water to make up to 3120 mL of water, and filter through a 0.22 μm filter membrane to obtain an aqueous solution of excipients. Finally, pour the sirolimus solution into the auxiliary material aqueous solution, and stir for one minute with a high-speed homogenizer at 10,000 rpm to obtain the sirolimus nanosuspension. The average particle size is 363nm as tested by a nanometer particle size analyzer. Embodiment 3: Preparation of medium concentration sirolimus nanosuspension (2mg / mL)
Embodiment 3
[0084] Sirolimus raw material and methylparaben were dissolved in 80 mL of ethanol at room temperature, and filtered through a 0.22 μm filter membrane to obtain a sirolimus solution. Then, dissolve povidone K30, sodium alginate, hydroxypropyl methylcellulose and sorbitol in 2500 mL of water at room temperature, add water to make up to 3120 mL of water, and filter through a 0.22 μm filter membrane to obtain an aqueous solution of excipients. Finally, pour the sirolimus solution into the auxiliary material aqueous solution, and stir for one minute with a high-speed homogenizer at 10,000 rpm to obtain the sirolimus nanosuspension. The average particle size is 363nm as tested by a nanometer particle size analyzer. Embodiment 3: Preparation of medium concentration sirolimus nanosuspension (2mg / mL)
[0085]
[0086]
[0087] Dissolve the sirolimus bulk drug in 40 mL of ethanol at room temperature, and filter through a 0.22 μm filter membrane to obtain a sirolimus solution. T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com