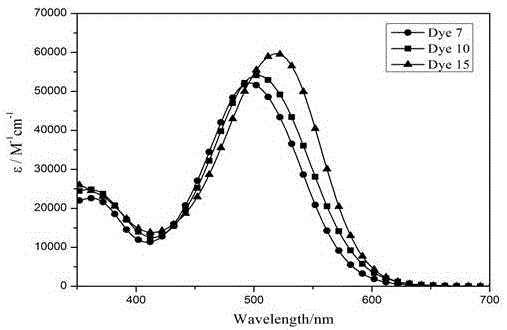
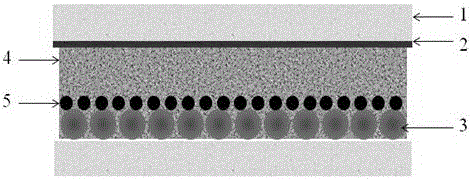
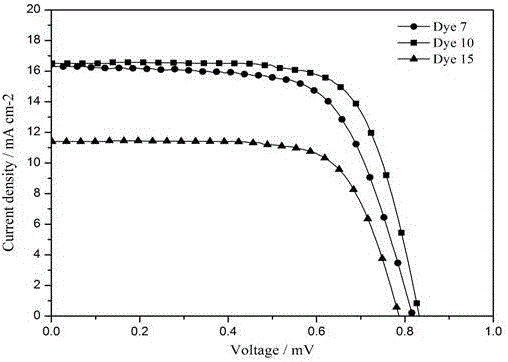
Organic dye based on tetrathienopyrrole as well as preparation method and application thereof
A technology of phenopyrrole and organic dyes, applied in the field of organic dyes and their preparation, can solve problems such as expensive preparation costs, environmental pollution restrictions, complex synthesis and separation processes, etc., and achieve improved photoelectric performance, high molar extinction coefficient, and spectral response range wide effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Preparation of organic dye 7
[0043] The synthetic route is as follows:
[0044]
[0045] The raw material 1 used in this example was prepared according to the literature Q. Yu, W. Fu, J. Wan, X. Wu, M. Shi, H. Chen. ACS Appl. Mater. Interfaces2014, 6, 5798−5809; 4- Hexyloxyaniline was prepared according to the literature Z.Wang, M. Liang, Y. Tan, L. Ouyang, Z. Sun, S. Xue. J. Mater. Chem. A, 2015,3, 4865–4874, raw material 5 and other reagents are available commercially.
[0046] Synthesis of Intermediate 2:
[0047]Into a 100 mL three-neck round bottom flask, add 0.604 g of raw material 1, 0.384 g of sodium tert-butoxide, 0.046 g of tris(dibenzylideneacetone) dipalladium, and 0.093 g of 1,1'-binaphthyl-2,2' -Bisdiphenylphosphine, 0.251 g 4-hexyloxyaniline and 15 mL of anhydrous toluene; under the protection of argon, the reaction system was heated to 110 °C for 8 h, and after the reaction solution was cooled to room temperature, the solvent was distilled off un...
Embodiment 2
[0056] Embodiment 2: the synthesis of organic dye 10
[0057] The synthetic route is as follows:
[0058]
[0059] The raw material 8 used in this example was prepared according to the literature Z. Wang, M. Liang, Y. Tan, L. Ouyang, Z. Sun, S. Xue. J. Mater. Chem. A, 2015, 3, 4865-4874 , Intermediate 4 was synthesized by referring to the preparation method in Example 1, and other reagents can be obtained commercially.
[0060] Synthesis of Intermediate 9:
[0061] Under the protection of argon, into a 100 mL three-neck round bottom flask, add 0.289 g raw material 5, 0.25 g intermediate 4, 0.464 g potassium carbonate, 0.019 g tetrakis(triphenylphosphine) palladium, 1.68 mL water and 10 mL Tetrahydrofuran; the reaction system was heated to 65°C for 8 h, the reaction solution was extracted with water and ethyl acetate, the organic phase was dried over anhydrous magnesium sulfate, concentrated by distillation under reduced pressure; the crude product was subjected to column ...
Embodiment 3
[0064] Embodiment 3: the synthesis of organic dye 15
[0065] The synthetic route is as follows:
[0066]
[0067] The raw material 1 used in this example can be synthesized by referring to the preparation method in Example 1, the raw material 8 can be synthesized by referring to the preparation method in Example 2, and the tripolyindene arylamine can be obtained by referring to the literature M. Liang, M. Lu, Q. Wang , W. Chen, H.Han, Z. Sun, S. Xue. Journal of Power Sources, 2011, 196, 1657–1664, and other reagents can be obtained commercially.
[0068] Synthesis of intermediate 11:
[0069] Into a 100 mL three-neck round bottom flask, add 0.604 g of raw material 1, 0.384 g of sodium tert-butoxide, 0.046 g of tris(dibenzylideneacetone) dipalladium, and 0.093 g of 1,1'-binaphthyl-2,2' - bis-diphenylphosphine, 1.12 g terdenarylamine, and 15 mL of anhydrous toluene; under the protection of argon, the reaction was heated to 110 °C for 8 h, the reaction system was cooled to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Short circuit current density | aaaaa | aaaaa |
| Short circuit current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com