L-AI (L-arabinose isomerase) and application thereof
An arabinose and isomerase technology, applied in the field of bioengineering, can solve problems such as restricting application and affecting the quality of D-tagatose, and achieving the effects of simple ingredients, large-scale industrial production, and mild reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: Escherichia coli genetic engineering strain E.coli BL21 (pETDuet-araA F279I ) of the acquisition.
[0036] (1) Acquisition of L-arabinose isomerase gene araA and construction of recombinant plasmid pETDuet-araA.
[0037] Design primers to introduce BamH I and EcoR I restriction sites that can be inserted into the multiple cloning site of the pETDuet-1 plasmid, the sequences are as follows:
[0038] araA.f: 5'-CGC GGATCC GATGTTGAAAATAAAAGA-3'
[0039] araA.r: 5'-CCG GAATTC TCAAATTTTTACAGTTT-3'
[0040]Using the genomic DNA of Bacillus coagulans as a template, PCR amplification was carried out using the above primers. The total PCR amplification system was 100 μL, containing 64 μL of ultrapure water, 20 μL of 5×FastPfu PCR Buffer, 0.25 mmol / L of dNTPs, 0.2-0.4 μmol / L of each primer, 50-200 ng of template, and FastPfu DNA Polymerase 5U of TransGen Company. PCR amplification conditions were: pre-denaturation at 95°C for 2 minutes, 30 cycles according t...
Embodiment 2
[0046] Example 2: Expression, purification and catalytic ability analysis of original L-AI and F279I.
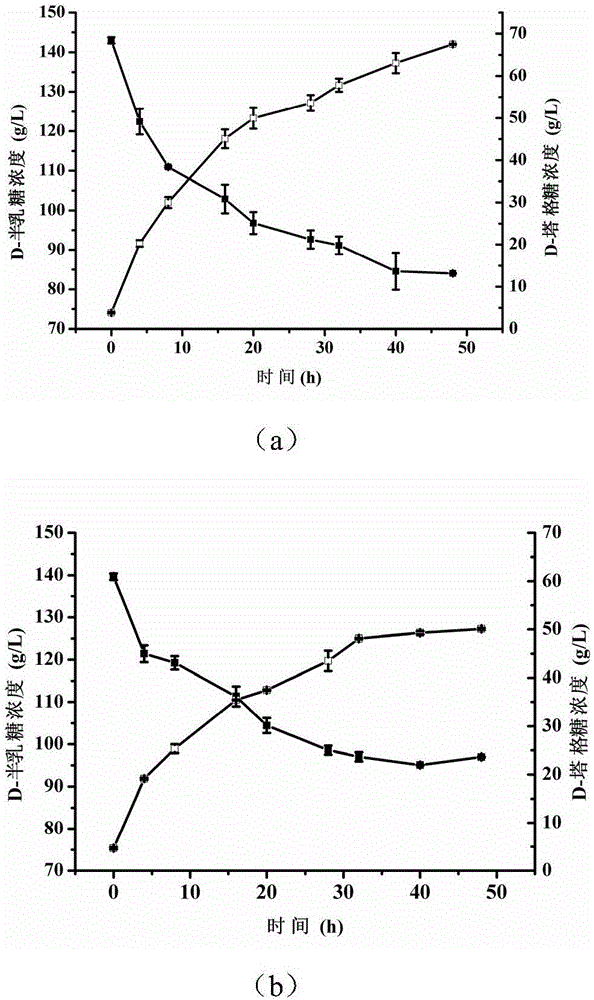
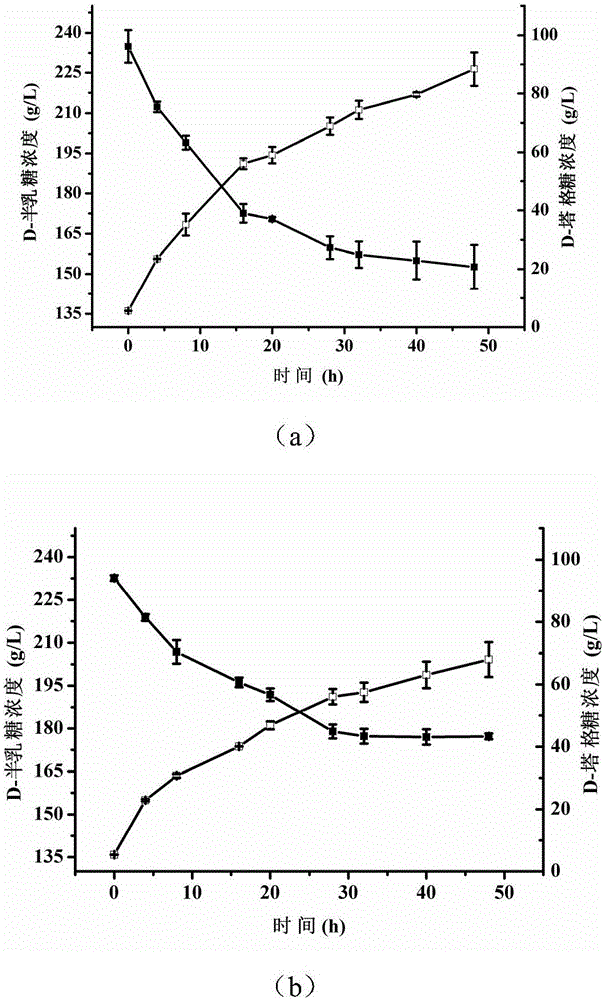
[0047] The recombinant bacterial strain E.coli BL21 (pETDuet-araA) that embodiment 1 obtains and E.coli BL21 (pETDuet-araA F279I ) were respectively inoculated in 50 mL of LB liquid medium containing 100 μg / mL ampicillin, cultured on a shaking table at 37°C for 12 hours, then transferred to 1L of LB liquid medium containing 100 μg / mL ampicillin, and cultured on a shaking table at 37°C. OD 600nm After reaching 0.4-0.8, add IPTG with a final concentration of 0.5-1 mmol / L to induce expression, and continue to culture at 20°C for 8 hours. After the induction culture, centrifuge at 12,000 rpm for 5 minutes to obtain cell pellets, wash twice with normal saline, and resuspend in 100 mL of phosphate buffer with pH 7.4 and 50 mmol / L. Ultrasound was used to break the cell wall in the ice-water mixture. The breaking time was 20 minutes. The solution after breaking the wall was centri...
Embodiment 3
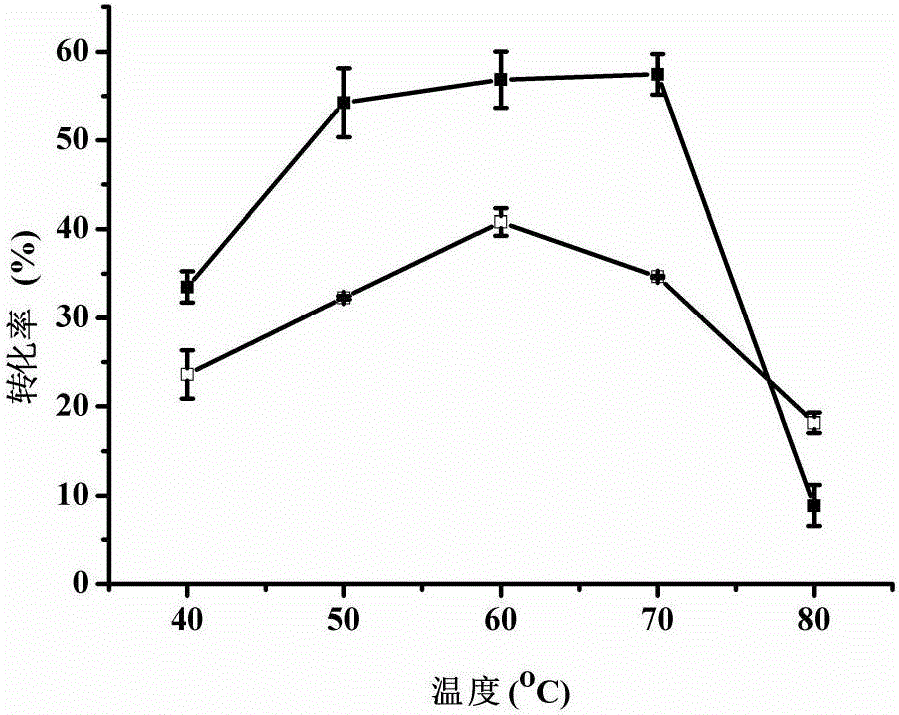
[0050] Example 3: E.coli BL21 (pETDuet-araA F279I ) and E.coli BL21(pETDuet-araA) produced D-tagatose at different temperatures.
[0051] In the 10mL reaction system, add 20g / L substrate D-galactose and recombinant Escherichia coli wet cells with a dry weight of 4.8g / L, mix well and shake the reaction at different temperatures. The pH of the reaction system is 7.5. After 15 hours, the reaction mixture was centrifuged to remove bacteria, and the supernatant was taken to detect the amount of D-tagatose produced. The results show( figure 1 ), when the reaction system temperature is 40~70℃, E.coli BL21(pETDuet-araA F279I ) was significantly higher than E.coli BL21(pETDuet-araA); in addition, E.coli BL21(pETDuet-araA F279I ) conversion within 50-70°C. In order to prevent browning reaction, 50°C can be selected for catalytic reaction; for E.coli BL21(pETDuet-araA), the optimum catalytic temperature is 60°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com