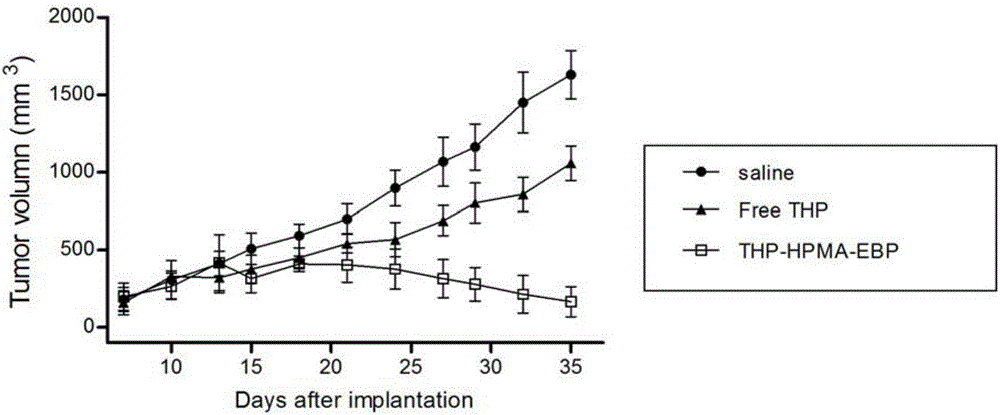
Tumor-targeted polypeptide-anthracycline derivative
A technology of tumor targeting and derivatives, applied in the direction of antineoplastic drugs, drug combinations, organic active ingredients, etc., can solve the problem of lack of tumor selectivity of anthracyclines, improve curative effect, increase drug concentration, reduce toxicity side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: Synthesis of N-methacryloylglycylglycine-EBP (Ma-GG-EBP)
[0023] Dissolve 90 mg of glycine (G)-glycine (G) dipeptide into 4 mL of water, add 400 mg of NaOH, add 70 μL of methacryloyl chloride dropwise while stirring at 0°C, adjust the pH to 2.0 with hydrochloric acid after reacting for 1 h, then add 2 mL of N, N-Dimethylformamide (DMF), add 126 mg of dicyclohexylcarbodiimide (DCC) at -15°C, stir for 3 hours, overnight at 4°C, centrifuge, filter to remove dicyclohexylurea crystals, and dry in vacuo To obtain methacryloylglycylglycine p-nitrophenyl ester (Ma-GG-ONp). Another 120 mg of EBP was dissolved in 4 mL of DMF, 30 mg of Ma-GG-ONp and pyridine were added, reacted at room temperature for 22 h, the reaction was terminated with 0.1 N NaOH, dialyzed with deionized water, and freeze-dried to obtain the product methacryloylglycyl Glycyl-EBP (Ma-GG-EBP).
Embodiment 2
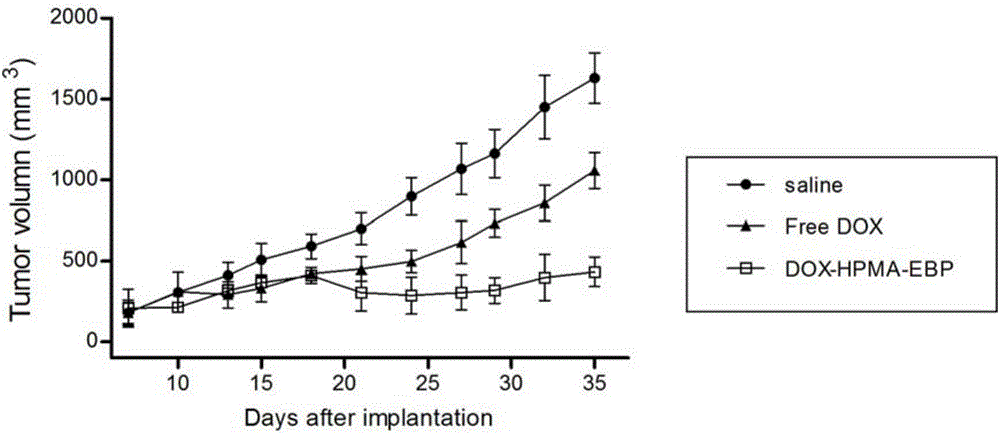
[0024] Embodiment 2: the synthesis of DOX-HPMA-EBP
[0025] Dissolve 200 mg of glycine (G)-phenylalanine (F)-leucine (L)-glycine (G) tetrapeptide into 10 mL of dichloromethane, add 5 mg of 4-(1,1,3,3-tetramethyl butyl) catechol and 32mg sodium carbonate, add 110μL methacryloyl chloride dropwise while stirring at 0°C, add 98mg hydrazine hydrate to react for 7h, elute with isopropane three times to remove unreacted hydrazine hydrate, add di Chloromethane / ethyl acetate crystallization to obtain methacryloylglycylphenylalanylleucylglycyl hydrazide (Ma-GFLG-NHNH 2), then add 8 mL of methanol to dissolve at room temperature, add 157 mg of doxorubicin (DOX) in the dark, add 420 μ L of acetic acid dropwise while stirring, react for 30 h, and purify by gel permeation chromatography to obtain the product methacryloylglycyl Phenylalanylleucylglycine doxorubicin (Ma-GFLG-DOX). Take another 148mg of HPMA, 42mg of Ma-GFLG-DOX, 30mg of Ma-GG-EBP and 1mg of azobisisobutyronitrile, add 2mL o...
Embodiment 3
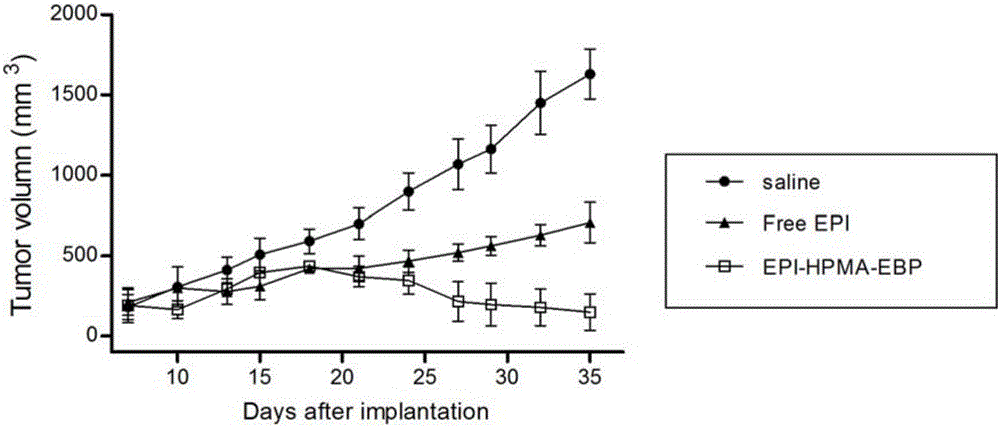
[0026] Embodiment 3: the synthesis of EPI-HPMA-EBP
[0027] Dissolve 200 mg of glycine (G)-phenylalanine (F)-leucine (L)-glycine (G) tetrapeptide into 10 mL of dichloromethane, add 5 mg of 4-(1,1,3,3-tetramethyl butyl) catechol and 32mg sodium carbonate, add 110μL methacryloyl chloride dropwise while stirring at 0°C, add 98mg hydrazine hydrate to react for 7h, elute with isopropane three times to remove unreacted hydrazine hydrate, add di Chloromethane / ethyl acetate crystallization to obtain methacryloylglycylphenylalanylleucylglycyl hydrazide (Ma-GFLG-NHNH 2 ), then add 8 mL of methanol to dissolve at room temperature, add 157 mg of epirubicin (EPI) in the dark, add 420 μL of acetic acid dropwise while stirring, react for 30 h, and purify by gel permeation chromatography to obtain the product methacryloylglycine Acylphenylalanylleucylglycine epirubicin (Ma-GFLG-EPI). Take another 148mg of HPMA, 42mg of Ma-GFLG-EPI, 30mg of Ma-GG-EBP and 1mg of azobisisobutyronitrile, add 2m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com