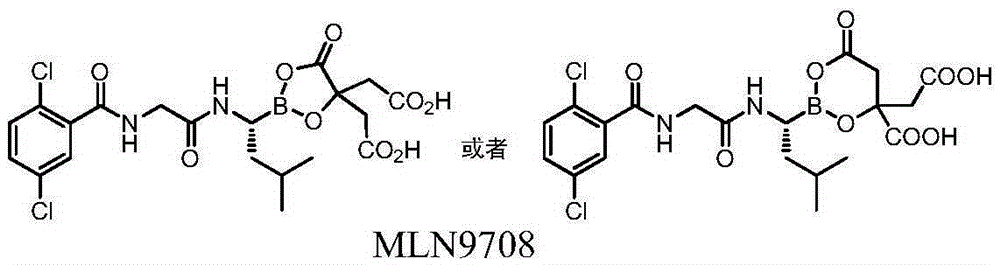
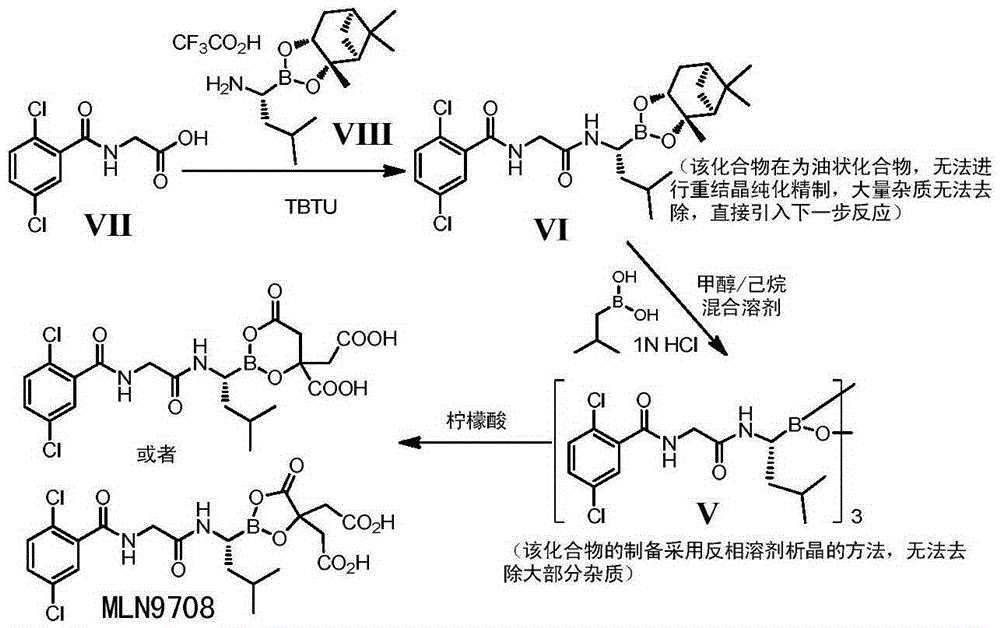
Boron-containing intermediate and application of same to medical industry
A technology for intermediates and boron compounds, which is applied to boron-containing intermediates and its application in the pharmaceutical industry, can solve the problems of compound V that is difficult to purify, cannot be purified, and does not reveal transformation, and achieves cheap and easy-to-obtain raw materials and purification Reduced difficulty and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 4-(R,S)-(carboxymethyl)-2-((R)-1-(2-(2,5-dichlorobenzamido)acetamido)-3-methylbutyl base)-6-oxo-1,3,2-dioxaborolane-4-carboxylic acid
[0044]
[0045] Step 1: N-2,5-dichlorobenzoyl-L-glycine methyl ester
[0046]
[0047] A mixture of 2,5-dichlorobenzoic acid (9.1 g, 47.6 mmol), L-glycine methyl ester hydrochloride (6.0 g, 47.5 mmol), TBTU (18.4 g, 57.3 mmol) and tetrahydrofuran (250 mL) was cooled to 0°C, stirred for 30min, and started to add diisopropylethylamine (23.6mL, 143.2mmol) dropwise. After the dropwise addition was completed, it was slowly raised to room temperature and reacted overnight. The complete reaction was monitored by TLC, the reaction solution was concentrated, and ethyl acetate was added, washed with saturated aqueous sodium bicarbonate solution, citric acid aqueous solution, water and saturated brine, dried over anhydrous sodium sulfate, filtered and concentrated to obtain N-2,5 -The crude product of dichlorobenzoyl-L-glycine m...
Embodiment 2
[0064]
[0065] A total of 5 g (11.3 mmol) of compound 1-6 obtained in Example 1 was dissolved in methanol (100 ml), and 4M KHF was added under room temperature 2 Aqueous solution (15ml), then stirred and reacted at room temperature for 2 hours, and TLC detected the reaction progress. After the reaction was completed, the solid was filtered and washed with methanol to obtain the solid compound I-b, which was dried under reduced pressure at 50°C to obtain the target solid compound I-a , the target product was recrystallized in methanol to obtain 3.9 g of the product (yield 84.7%).
[0066]A total of 3g (7.3mmol) of the compound I-b obtained above was dispersed and dissolved in acetonitrile (25ml) to form a suspension, then triethylamine (5ml) was added, and an ethyl acetate solution of citric acid (1.4g, 7.3mmol) was added , the above mixed solution was stirred and reacted at room temperature for 30 minutes. At room temperature, 5.3 g of silicon tetrachloride was added, and ...
Embodiment 3
[0068]
[0069] Compound 1-6 was prepared according to Example 1 to obtain a total of 10 g (22.5 mmol), ethyl acetate (100 ml) and N-methyliminodiacetic acid (compound 3-1, 3.31 g, 22.5 mmol) were added, and stirred overnight at room temperature, Filter, wash with ethyl acetate, and dry to obtain 9.5 g of the target product with a yield of 89.4%.
[0070] A total of 9 g (19.0 mmol) of compound 3-2 obtained above was dissolved in methanol (100 ml), and 4M KHF was added at room temperature 2 Aqueous solution (15ml), then stirred and reacted at room temperature for 2 hours, TLC detected the reaction progress, after the reaction was completed, filtered the solid, washed with methanol to obtain solid compound I-a, and dried under reduced pressure at 50°C to obtain the target solid product compound I-a , the target product was recrystallized in methanol to obtain 6.9 g of the product (yield 85.9%).
[0071] A total of 6g (14.2mmol) of the compound I-a obtained above was disperse...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com