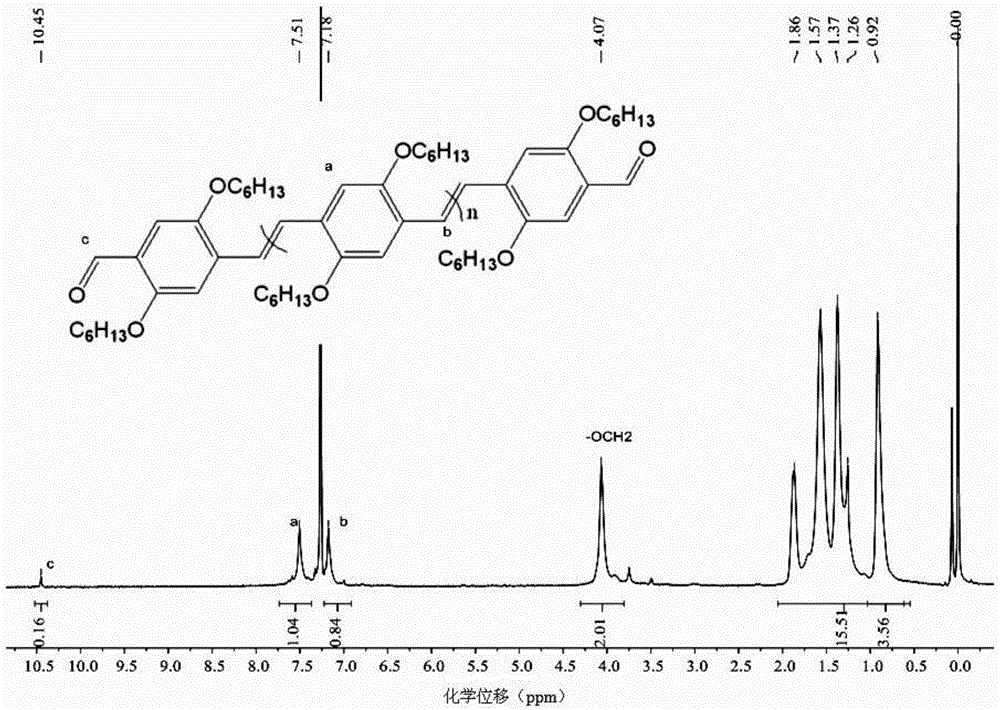
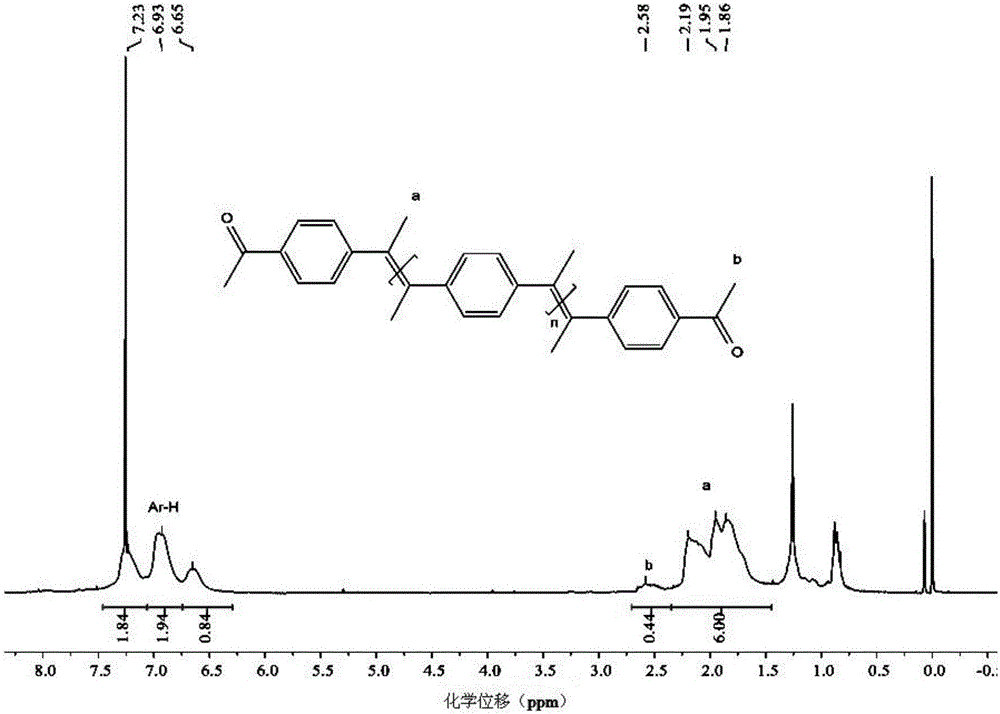
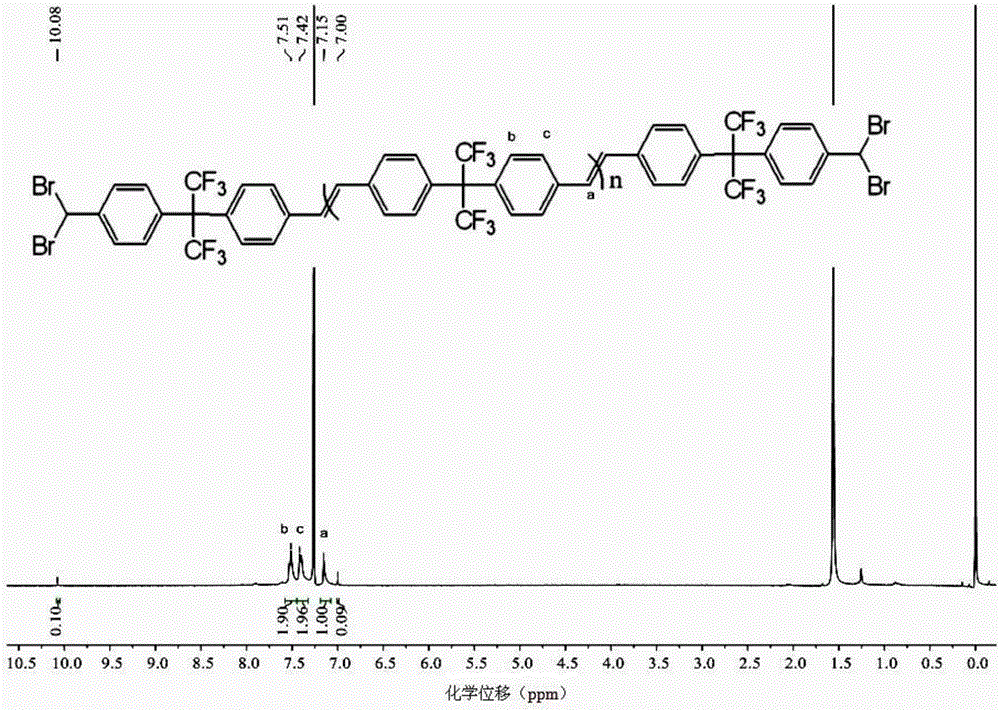
Polymer synthesis method using carbon-carbon double bond construction
A synthesis method and technology of carbon double bonds, applied in the field of polymer preparation, can solve problems such as harsh reaction conditions, and achieve the effects of mild synthesis conditions, convenient synthesis, and variable structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] (1) Preparation of monomer
[0036] All bis-dibromo monomers can be prepared by the existing common synthetic methods. The molecular structures of bis-dibromo compounds used in the examples are shown in the following and Table 1.
[0037]
[0038] Table 1 Different dibromo compound monomers and their abbreviations
[0039] Monomer abbreviation Monomer name A1
1,4-bis(dibromomethyl)benzene A2
Ethyl 2,5-bis(dibromomethyl)benzoate A3
2,5-bis(dibromomethyl)phenol acetate A4
2,5-bis(dibromomethyl)phenylhexyl ether B1
2,5-Dihexyloxy-1,4-bis(dibromomethyl)benzene B2
2,5-Dioctyloxy-1,4-bis(dibromomethyl)benzene C1
1,4-bis(dibromoethyl)benzene D1
3,5-bis(dibromomethyl)anisole D2
3,5-bis(dibromomethyl)tert-butylbenzene D3
Ethyl 3,5-bis(dibromomethyl)benzoate E1
2,2-bis(4-dibromomethylphenyl)hexafluoropropane E2
4,4’-Bis(dibromomethyl)phenyl ketone E3
4,4’-Bis(dibromomethyl)phenylsulfone
[0040] (2) Polymerization method
[0041] Deoxidize a certain stoichiometric ratio of bis-dibrom...
Embodiment 1
[0046] Example 1 Synthesis of TPMA
[0047] 16.4g of 2-chloromethylpyridine hydrochloride was dissolved in 40mL of deionized water, cooled in an ice bath, and 20mL of 5M NaOH aqueous solution was slowly added, and the solution turned pink. Add 80mL CH containing 5.4g 2-(aminomethyl)pyridine 2 Cl 2 The solution was warmed to room temperature. Use a micro syringe to add 20 mL of 5M NaOH aqueous solution, and drop it in 50 hours. Stop the reaction, wash the organic phase with 3×10 mL 15% NaOH aqueous solution, combine the organic phases, anhydrous MgSO 4 Dry, filter, and concentrate. Extract the product with ether under boiling state, remove insoluble matter, cool, crystallize the product in ether, and filter. Continue recrystallization 3 times to obtain pale yellow needle-like crystals with a yield of 37%. 1 H NMR(400MHz, CDCl 3 ): 8.54-8.53(d,3H), 7.67-7.64(t,3H), 7.60-7.58(d,3H), 7.16-7.13(t,3H), 3.89(s,6H).
Embodiment 2
[0048] Example 2 Monomer A 1 Synthesis
[0049] 11.7 g of N-bromosuccinimide, 0.3 g of benzoyl peroxide, 4.7 mL of p-xylene, and 50 mL of carbon tetrachloride were sequentially added to a 100 mL three-necked flask, and nitrogen was bubbled for 10 minutes, and refluxed for 1.5 hours. The reaction was stopped, filtered, and the filtrate was concentrated to obtain a pale yellow solid. The crude product was recrystallized with n-hexane to obtain white crystals, which were dried in vacuum with a yield of 60%. 1 H NMR(400MHz, CDCl 3 ):
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com