Polyaryletherketone containing indole group and preparation method of polyaryletherketone
A polyaryletherketone, indole group technology, applied in the field of polymer compounds and their preparation, can solve problems such as poor solubility, low glass transition temperature, etc., and achieve high glass transition temperature, good thermal stability, The effect of good dissolving properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
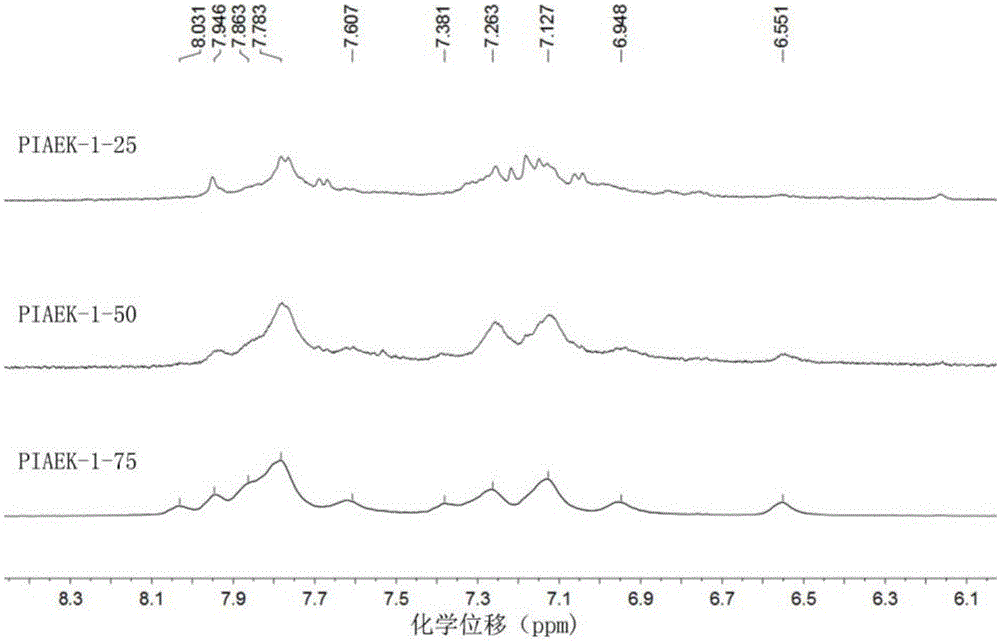
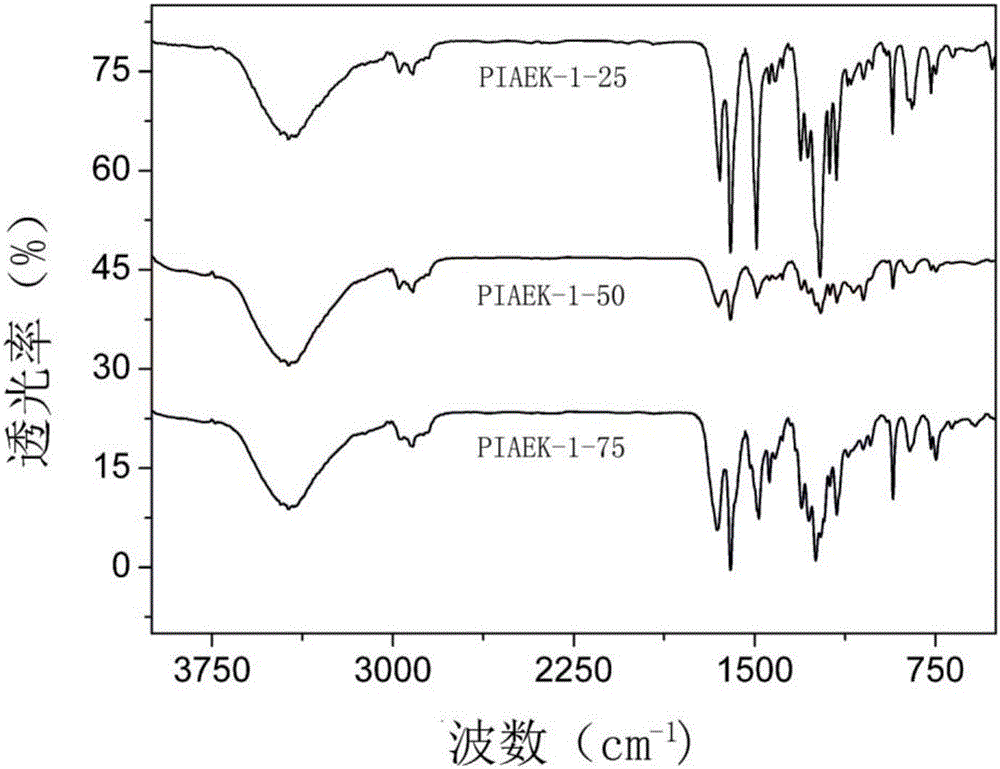
[0033] Under nitrogen protection, add a mmol 4-oxindole, b mmol hydroquinone and (a+b)mmol 4 , 4'-difluorobenzophenone, using 1.8(a+b)mmol anhydrous potassium carbonate as a base to make a salt, using N-methylpyrrolidone (amount of 15mL) as a solvent, and toluene (amount of 3mL) as a Water agent, heat up to 140°C and react with water for 3 hours, then distill off toluene, then rapidly raise the temperature to 180°C to continue the reaction for 4 hours; then slowly cool the reaction system to room temperature, pour the reaction solution into deionized water for precipitation, suction filter, and then After extraction with methanol for 10 hours; the extract was then dried in vacuum (at a pressure of 0.1 MPa) at 100°C for 10 hours to obtain the target polymer—polyhydroquinone monocarbonyl aryl ether ketone containing indole groups. The rate is above 92%. By adjusting the ratio of a and b (the specific amounts of a and b are shown in Table 1), the content of indole groups in the ...
Embodiment 2
[0039] Under nitrogen protection, add a mmol 4-oxindole, b mmol hydroquinone and (a+b)mmol 4 , 4'-bis(4-fluoro-benzoyl)benzene, using 1.8(a+b)mmol anhydrous potassium carbonate as a base to form a salt, using N-methylpyrrolidone (amount of 20mL) as a solvent, and toluene ( The dosage is 4mL) as a water-carrying agent, heat up to 140°C and react with water for 4 hours, then distill off toluene, then rapidly raise the temperature to 180°C to continue the reaction for 6 hours; then slowly cool the reaction system to room temperature, and pour the reaction solution into deionized water for precipitation , suction filtration, and then extracted with methanol for 18 hours; the extract was then dried in vacuum (at a pressure of 0.1 MPa) at 100°C for 10 hours to obtain the target polymer - polyhydroquinone p-dicarbonyl containing indole groups The yield of aryl ether ketone is more than 90%. By adjusting the ratio of a and b (the specific amounts of a and b are shown in Table 2), the...
Embodiment 3
[0044] Under nitrogen protection, add a mmol 4-oxindole, b mmol hydroquinone and (a+b)mmol 1 ,3-bis(4-fluoro-benzoyl)benzene, use 1.8(a+b)mmol anhydrous potassium carbonate as a base to form a salt agent, use N-methylpyrrolidone (amount of 20mL) as a solvent, and use toluene (amount 2mL) is a water-carrying agent, heat up to 140°C and react with water for 4 hours, then distill off the toluene, and then quickly heat up to 180°C to continue the reaction for 6 hours; then slowly cool the reaction system to room temperature, and pour the reaction solution into deionized water for precipitation. Suction filtration, and then extracted with methanol for 10 h; the extract was then dried in vacuum (at a pressure of 0.1 MPa) at 100°C for 10 h to obtain the target polymer—polyhydroquinone meta-dicarbonyl aromatic polyhydroquinone containing indole groups. Ether ketone, its productive rate is above 90%. By adjusting the ratio of a and b (the specific amounts of a and b are shown in Table...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com