Double-chamber bag amino acid peritoneal dialysis solution and preparation method thereof
A peritoneal dialysate, amino acid technology, applied in blood diseases, pharmaceutical formulations, extracellular fluid diseases, etc., to reduce the risk of aggravating acidosis, reduce abdominal pain, and reduce the effect of content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
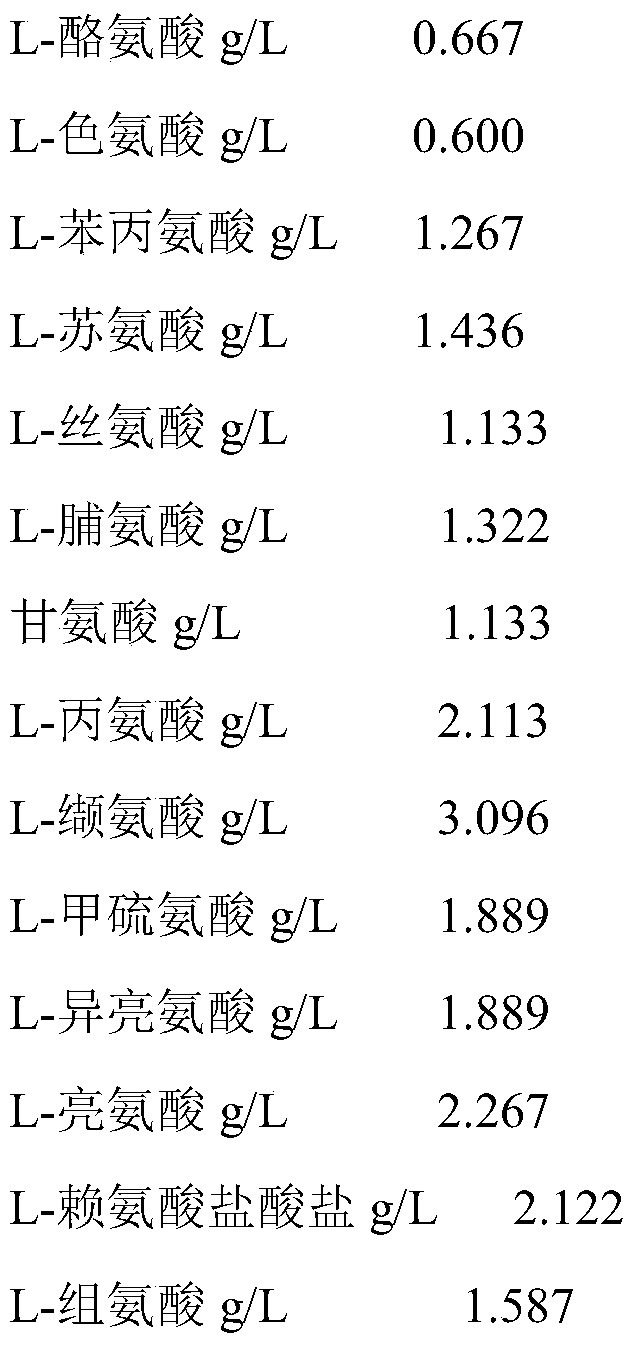
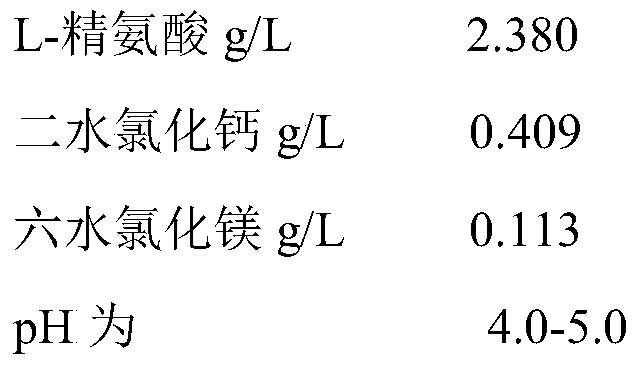
[0028] Amino Acid Indoor Composition (First Chamber)
[0029]
[0030]
[0031] Buffer chamber composition (second chamber)
[0032]
[0033] Amino acid and ion composition in the mixture
[0034]
Embodiment 2
[0036] Amino Acid Indoor Composition (First Chamber)
[0037]
[0038] Buffer chamber composition (second chamber)
[0039]
[0040] Amino acid and ion composition in the mixture
[0041]
[0042]
[0043] The preparation method is as follows
[0044] 1. Preparation of the first chamber solution:
[0045] Add fresh water for injection at 50-60°C and about 50-70% of the prescription amount into the preparation tank, turn on the stirring paddle, put in various amino acids, calcium chloride dihydrate, and magnesium chloride hexahydrate in order to dissolve, and stir thoroughly for 10-10 ~ After 15 minutes, after it is completely dissolved, add water for injection to a sufficient amount, measure the pH, stir well, and take a sample to detect the intermediate. After passing the test, turn on the liquid medicine pump to filter the liquid medicine through the filter element respectively.
[0046] 2. Preparation of the second chamber solution:
[0047] Add fresh water...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com