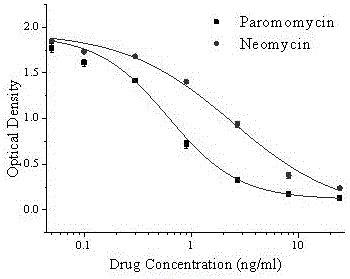
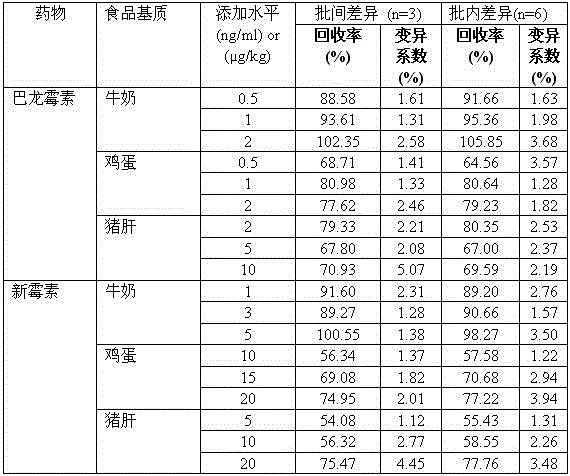
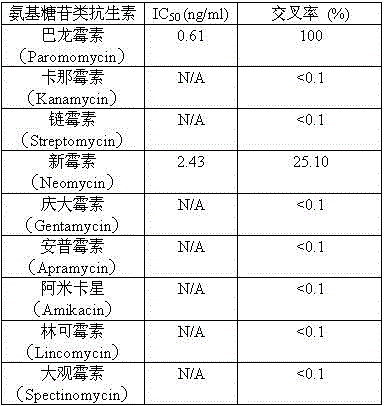
Hybridoma cell line C1 capable of secreting anti-paromomycin monoclonal antibody and application of hybridoma cell line C1
A hybridoma cell line and a paromomycin-resistant technology, applied in the field of immunochemistry, can solve the problem of no paromomycin ELISA method and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Example 1: Synthesis of Immunogens and Coating Gens
[0014] The immunogen was coupled by the glutaraldehyde method: 31.96 mg of paromomycin was dissolved in 4 mL of 0.01 M phosphate buffer solution, 200 μL of 1% glutaraldehyde aqueous solution was added and stirred for 15 min. 20 mg of bovine serum albumin was dissolved in 2 mL of 0.01M phosphate buffer solution, reacted at 4°C for 1 h, added an appropriate amount of sodium borohydride to terminate the reaction, and incubated at 4°C for 2 h. The reaction mixture was dialyzed to remove free paromomycin to obtain the immunogen. The coating was synthesized by the carbodiimide method: 10 mg ovalbumin was dissolved in 1 mL pH 4.7 0.1 M MES (morpholine ethanesulfonic acid) buffer solution, 2 mg EDC and 1.2 mg NHS were added, and reacted at room temperature for 2 h. 19.03 mg paromomycin dissolved in 1 mL 0.05M KH 2 PO 4 , the activated protein solution was slowly added dropwise into the paromomycin solution, and reacted fo...
Embodiment 2
[0015] Example 2: Immunization of mice
[0016] The immunogen was diluted to an appropriate concentration with physiological saline, emulsified with an equal volume of Freund's adjuvant, and injected at multiple points on the back of 6-8 week-old BALB / c mice. For the first immunization, complete Freund's adjuvant was used at a dose of 100 μg. The following four booster immunizations used incomplete Freund's adjuvant, the immunization dose was 50 μg, and the immunization interval was 21 days. After the third immunization, blood was collected from the tail vein of the mice, and the sera were tested by indirect ELISA. After the fifth immunization, the mice with high titer and inhibition were screened for intraperitoneal immunization, and the spleens of the mice were taken out three days later for cell fusion.
Embodiment 3
[0017] Example 3: Cell Fusion and Screening
[0018] Mouse splenocytes and tumor cells were fused under the action of PEG 1500 to form hybridoma cells. Detection was carried out on the seventh day after fusion, and the cell lines with high titer and good paromomycin recognition were screened for subcloning, and so on, 5 times of subcloning were performed to obtain pure cell lines.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com