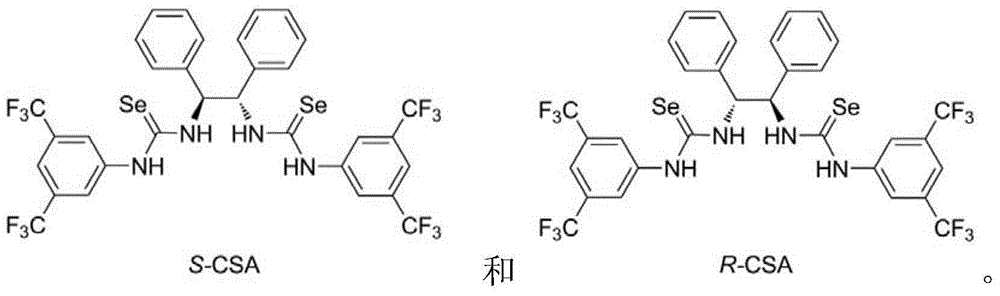
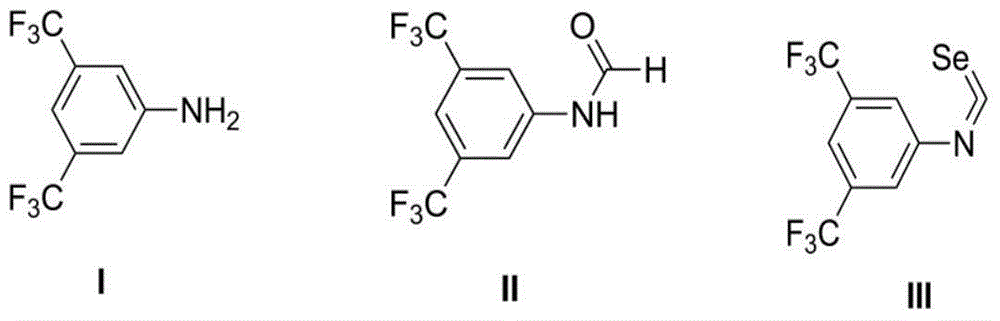
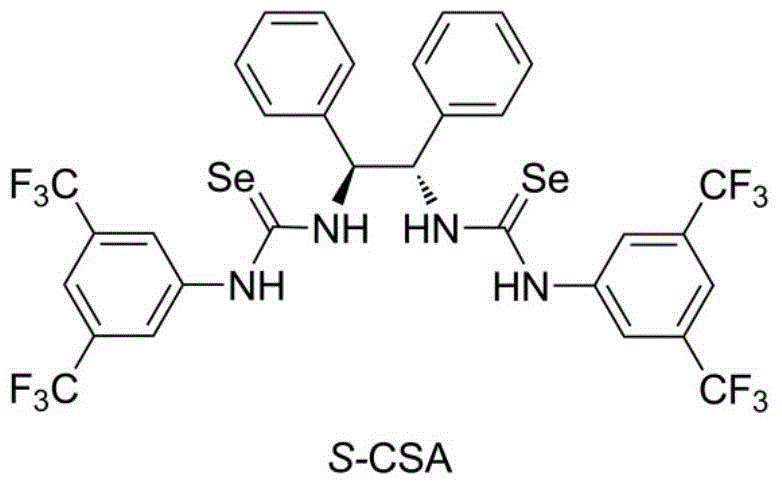
Chiral selenourea compound, preparation and application thereof
A chiral compound and compound technology, applied in organic chemistry, analysis by nuclear magnetic resonance, etc., can solve the problem of rare reports of chiral selenourea, and achieve the effect of large chemical shift difference and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] The raw material used in the preparation of the chiral selenourea reagent is 3,5-ditrifluoromethylaniline, the reagent used for formylation is formic acid, the catalyst is zinc chloride, and the reaction temperature can be between room temperature and 90 degrees. The reaction time is 3-24 hours; the reagent used in the dehydrogenation of the formylation product is triphosgene or phosgene, the reagent used in the selenogenesis is selenium powder, and the solvent can be one or more of dry dichloromethane, chloroform, tetrahydrofuran, etc. Its mixture, the reaction temperature is the reflux temperature of the solvent, the reaction time is 3-8 hours before adding the selenium powder, and 12-24 hours after adding the selenium powder, the reaction should be completed in the dark, and the product isoselenocyanate should pass through the column layer. It is suggested that the elution solvent can be dry n-hexane, n-pentane, petroleum ether, etc.; the condensation one-step reactio...
example 1
[0024] Example 1 Synthesis of intermediate 3,5-ditrifluoromethylphenylisoselenocyanate
[0025] Add 10g of 3,5-ditrifluoromethylaniline and 1.2g of anhydrous zinc chloride to a 50mL round-bottomed flask, add 10mL of formic acid dropwise at room temperature, continue stirring at room temperature for 10 minutes after the addition, and then heat the mixture to 70 degrees The reaction is about 5 hours. The reaction mixture was cooled to room temperature, diluted with 40 mL of ethyl acetate, washed with water and brine successively, dried over anhydrous sodium sulfate, and the crude product concentrated under reduced pressure was recrystallized from anhydrous ethanol to obtain 10.66 g of formamide.
[0026] Dissolve 1 g of formamide in dry dichloromethane, add 2.25 mL of triethylamine and 2 g of 4A molecular sieves, dropwise add 0.7 g of triphosgene in dichloromethane solution under reflux, and continue to heat and reflux for 4 hours after the dropwise addition Then it was cooled ...
example 21
[0027] Example 21, Synthesis of 2-diphenylethylenediamine-derived diselenourea chiral reagent
[0028] Dissolve 0.21g (S,S)-1,2-diphenylethylenediamine in dry dichloromethane, add 0.64g of 3,5-ditrifluoromethylphenylisoselenocyanate, and the reaction mixture is at room temperature The mixture was stirred overnight in the dark, and then dry n-hexane was added to dilute the reaction solution. A large amount of solid was precipitated. After filtration, washing with n-hexane and vacuum drying, 0.81 g of a white solid product was obtained. Mp 165-166°C; [α] 30 D =31.8(c=1.0in CHCl 3 ); 1 H NMR (400MHz, CDCl 3 ): δ=8.84(br s, 2H), 7.95(br s, 2H), 7.73-7.67(m, 6H), 7.40-7.20(m, 10H), 6.17(s, 2H) ppm; 13 C NMR (100MHz, CDCl 3 ): δ=179.9, 138.1, 136.4, 133.9, 133.6, 133.2, 132.9, 129.3, 128.9, 127.8, 126.8, 124.8, 124.1, 121.4, 120.7, 118.7, 67.6ppm; 77 Se NMR (95MHz, CDCl 3 ): δ=247; ESI-MS: calcd for C32H22F12N4Se2+H 851.0062, found 851.0073.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com