Novel antioxidant and preparation method thereof
An antioxidant, a new type of technology, applied in the direction of food ingredients as antioxidants, carbon-based compound preparation, chemical instruments and methods, etc., can solve the limitation of natural antioxidant antioxidant effect, natural antioxidant antioxidant effect and structure are not very deep and other problems, to meet the requirements, high boiling point, strong antioxidant activity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: concrete steps are as follows:
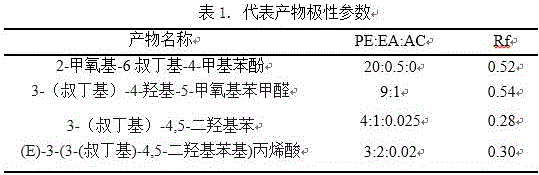
[0031] a. in N 2 protection, 7.8 ml compound 2-methoxy-4-methylphenol was added to 19 ml 85% H 3 PO 4 , stirred for 5 minutes, controlled the temperature at 75°C, added 6.5ml of tert-butanol, dried over anhydrous sodium sulfate, and rotary evaporated to obtain a bright yellow oily liquid. The crude product was subjected to column chromatography, followed by petroleum ether, petroleum ether: ethyl acetate = 20 :0.5 to separate a colorless oily liquid with a yield of 70%, product: 2-methoxy-6-tert-butyl-4-methylphenol.
[0032] 1 HNMR(500M, CDCl3)δ1.49 (s, 9H), 2.37 (s, 3H), 3.93 (s, 3H), 5.91(s,1H), 6.67 (s, 1H), 6.78(d, J=0.35 Hz.2H).
[0033] b.N 2 Protection, 0°C, ice-water bath, dissolve 3ml of the compound 2-methoxy-6-tert-butyl-4-methylphenol in 20ml of tert-butanol, stir well, add 2ml of liquid bromine dropwise (with a 5ml glass syringe, plastic needle slowly Add dropwise for 10 minutes, keep the solution light...
Embodiment 2
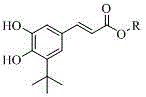
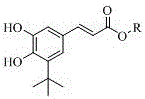
[0039] Example 2: The specific steps are: dissolve 1 gram of (E)-3-(3-(tert-butyl)-4,5-dihydroxyphenyl)acrylic acid obtained in Example 1 in 70ml of ethanol, add 2ml of concentrated Sulfuric acid solution, heated to reflux at 90°C for 1h, cooled at room temperature. Rotary evaporation at 40°C, remove most of the methanol, add water, extract with ethyl acetate, the organic phase is sequentially washed with NaHCO 3 , washed with saturated brine, Na 2 SO 4 Drying, spin-drying, ethanol crystallization, (E)-3-(3-(tert-butyl)-4,5-dihydroxyphenyl) ethyl acrylate was obtained with a yield of 95%.
Embodiment 3
[0040] Embodiment 3: The specific steps are: 1 gram of (E)-3-(3-(tert-butyl)-4,5-dihydroxyphenyl)acrylic acid obtained in Example 1 is dissolved in hot 50ml lauryl alcohol, Add 2ml of concentrated sulfuric acid, heat to reflux at 90°C for 1h, and cool at room temperature. Rotary evaporation at 100°C, remove most of the lauryl alcohol, add water, extract with ethyl acetate, the organic phase is successively washed with NaHCO 3 , washed with saturated brine, Na 2 SO 4 Drying, spin-drying, and crystallization of benzene gave (E)-3-(3-(tert-butyl)-4,5-dihydroxyphenyl)ethyl acrylate with a yield of 95%.
[0041] (E)-3-(3-(tert-butyl)-4,5-dihydroxyphenyl)acrylic acid obtained by the present invention and C 1 -C 22 Alcohols can undergo esterification reaction to form the corresponding (E)-3-(3-(tert-butyl)-4,5-dihydroxyphenyl)acrylate, the reaction conditions are similar, and will not be described in detail here.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com