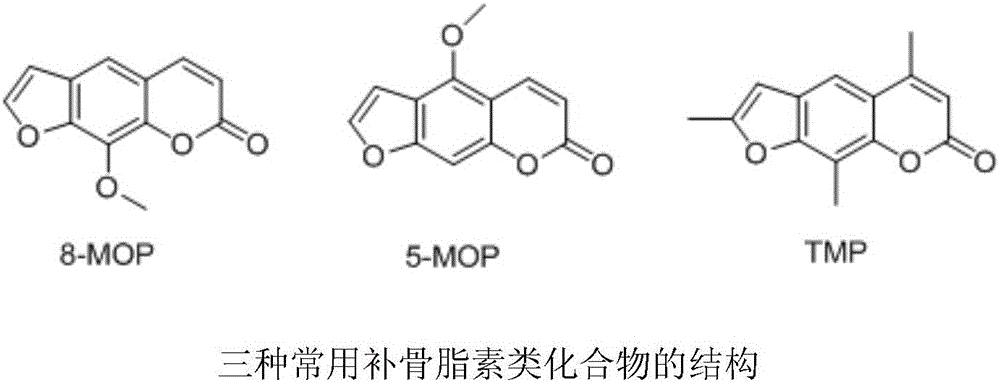
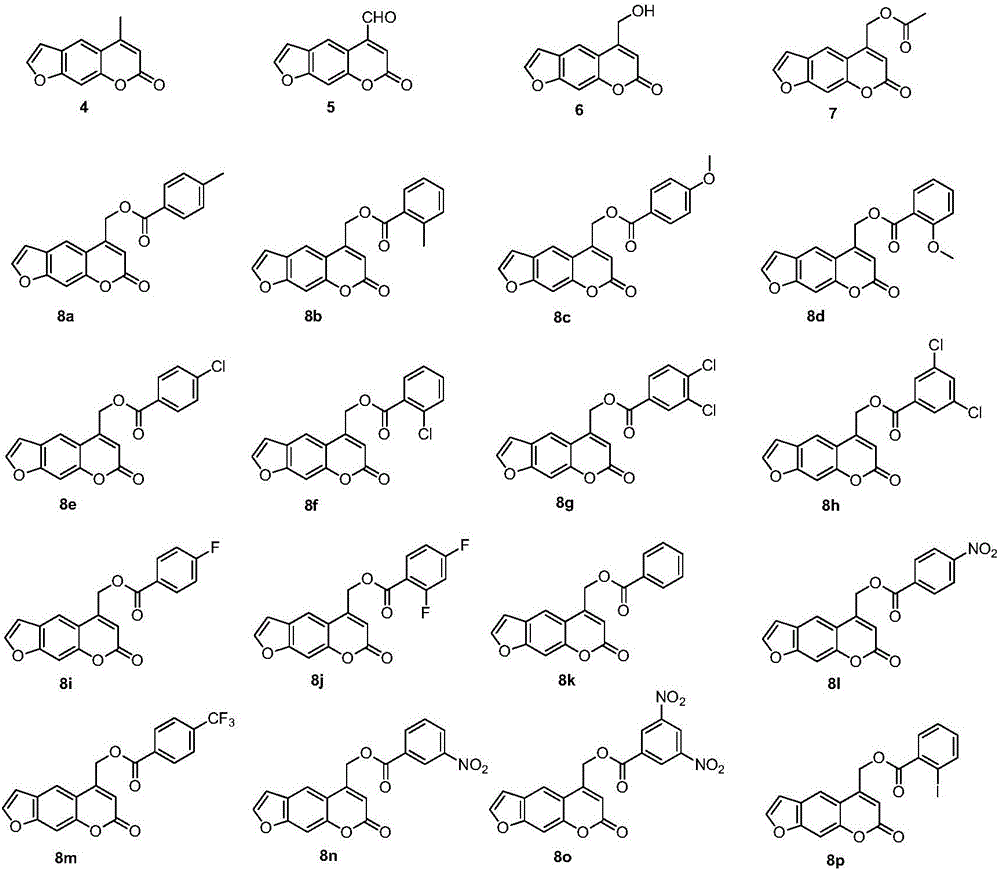
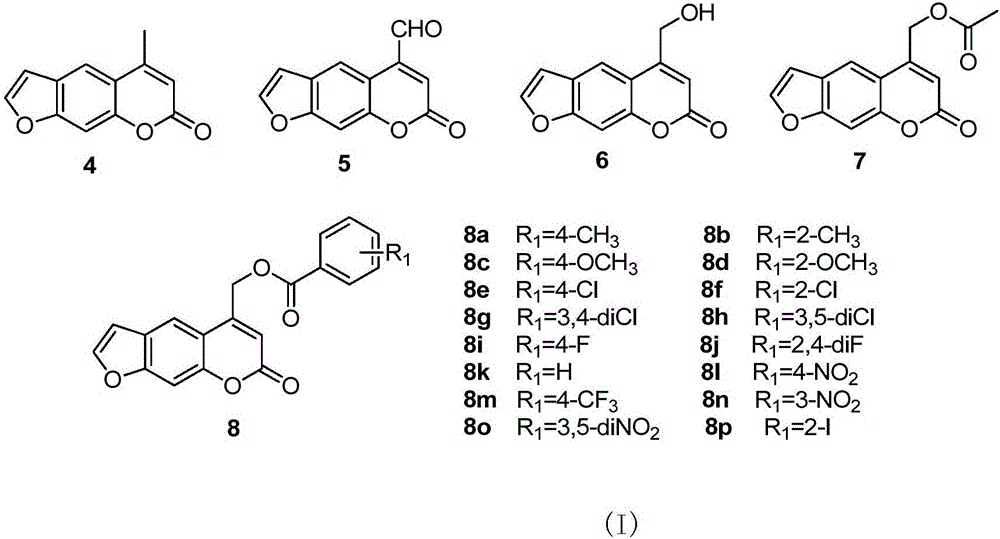
Psoralen ester derivatives and applications thereof
A technology of psoralen and its derivatives, which can be applied in drug combinations, skin diseases, and vector-borne diseases, etc. It can solve the problems of weak material basis research, unclear mechanism of action and metabolic process in vivo and in vitro, and constraints on secondary development, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Preparation of intermediate 2:
[0088] At room temperature, dissolve 1.76g (10mmol) of intermediate 1 and 2.76g (20mmol) of potassium carbonate in 50mL of acetone, then add 1.20g (15mmol) of 2-chloroethanol, heat to reflux and react until all the raw materials disappear , stop the reaction, cool to room temperature, filter, the filtrate is concentrated, the residue is eluted by column chromatography gradient, the eluent is petroleum ether: ethyl acetate with a volume ratio of 1:1, and 2.10g of intermediate 2,4- Methyl-7-hydroxyethoxycoumarin;
[0089] NMR data of 4-methyl-7-hydroxyethoxycoumarin (intermediate 2):
[0090] 1 H NMR (400MHz, CDCl 3 )d 7.51(d, J=9.0Hz, 1H), 6.91–6.83(m, 2H), 6.15(d, J=1.1Hz, 1H), 4.15(t, J=8.7Hz, 2H), 4.01(m ,2H),2.40(d,J=1.1Hz,3H).
[0091] Preparation of intermediate 3:
[0092] Under low-temperature reaction conditions at a temperature of -78°C, dissolve 50.25 g (2 mmol) of oxalyl chloride in a small amount of dry dichloromethane,...
Embodiment 2
[0117] Preparation of compound 8b:
[0118] The preparation of compound 2-compound 7 is carried out according to embodiment 1:
[0119] Under ice-bath conditions, 0.22g (1mmol) compound 6, 0.21g (1mmol) dicyclohexylcarbodiimide (DCC), 0.12g (1mmol) 4-dimethylaminopyridine (DMAP) and 0.16g (1.2 mmol) 2-methylbenzoic acid was dissolved in an appropriate amount of dichloromethane, fully stirred to make it completely dissolved, moved to room temperature to continue the reaction, after all the raw materials disappeared, filtered, and the mother liquor was successively washed with 1M hydrochloric acid solution and saturated hydrogen carbonate The sodium solution was washed, dried and concentrated, and the residue was eluted by gradient column chromatography, and the eluent was petroleum ether:ethyl acetate at a volume ratio of 15:1, to obtain 0.31g of compound 8b, (7-oxo-7H- Furo[3,2-g]benzopyran-5-)methyl-(2-methyl)benzoate;
[0120] NMR data of (7-oxo-7H-furo[3,2-g]benzopyran-5-...
Embodiment 3
[0124] Preparation of compound 8c:
[0125] Compound 2-Compound 7 were prepared according to Example 1.
[0126] Under ice-bath conditions, 0.22g (1mmol) compound 6, 0.21g (1mmol) dicyclohexylcarbodiimide (DCC), 0.12g (1mmol) 4-dimethylaminopyridine (DMAP) and 0.18g (1.2 mmol) 4-methoxybenzoic acid was dissolved in an appropriate amount of dichloromethane, fully stirred to make it completely dissolved, moved to room temperature to continue the reaction, after all the raw materials disappeared, filtered, and the mother liquor was successively washed with 1M hydrochloric acid solution and saturated carbonic acid Sodium hydrogen solution was washed, dried, concentrated, and the residue was eluted by gradient column chromatography, and the eluent was petroleum ether:ethyl acetate with a volume ratio of 15:1, to obtain 0.32g of compound 8c, (7-oxo-7H - Furo[3,2-g]benzopyran-5-)methyl-(4-methoxy)benzoate;
[0127] NMR data of (7-oxo-7H-furo[3,2-g]benzopyran-5-)methyl-(4-methoxy)be...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com