Dye sensitizer molecule taking triazole as core and preparation method of dye sensitizer molecule
A technology of dye sensitizer and molecule, which is applied in the field of dye sensitizer molecule and its preparation, can solve the problem that the photoelectric conversion efficiency is not very high, and achieve the effect of wide absorption band
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
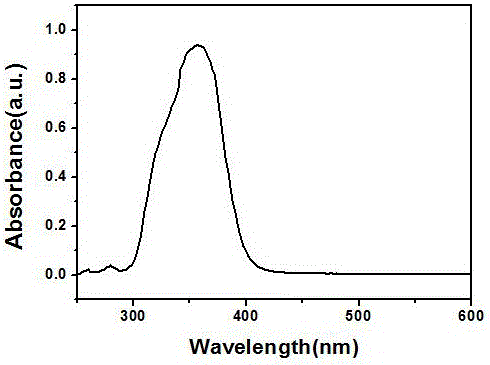
Image
Examples
Embodiment 1
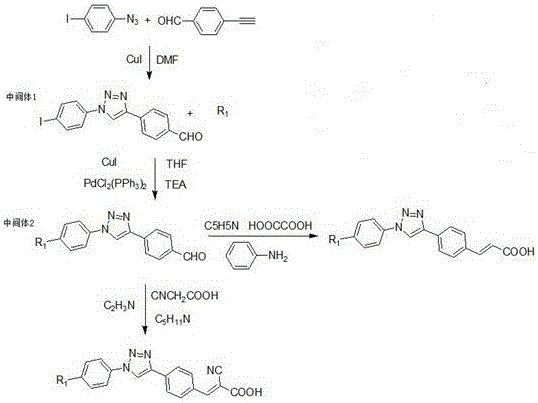
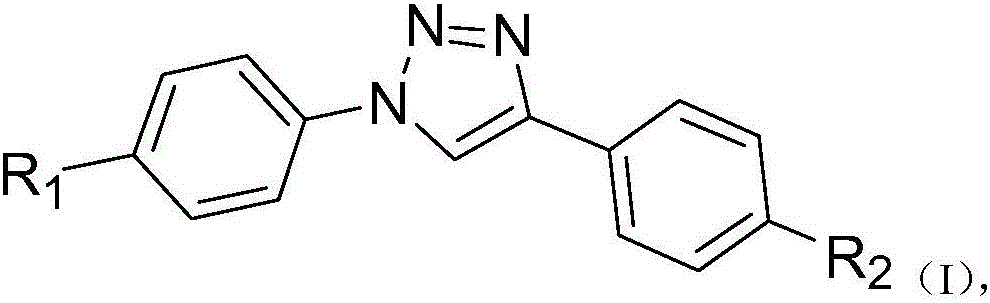
[0040] What this example prepares is the dye molecule described in general formula, wherein R1 is where n=12, R2 is The reaction steps are as follows:
[0041] (1) Synthesis of p-iodophenylazide:
[0042] Dissolve 14.4mmol (3.15g) p-iodoaniline in 9.1ml hydrochloric acid aqueous solution (HCl: water = 1:1), and place in a 100ml small beaker, stir at 0 degrees Celsius, 14.4mmol (1.00g) sodium nitrite Dissolved in 51.3ml of ice water, and added dropwise to the reaction beaker, 14.4mmol (0.94g) of sodium azide was dissolved in 11.3ml of ice water, then added dropwise to the reaction vessel, and reacted for 30 minutes. The product was extracted with dichloromethane, and the organic layer was dried with 2.00 g of anhydrous magnesium sulfate. The magnesium sulfate was filtered out and then rotary evaporated. The product was placed in a vacuum oven and dried to obtain 3.29 g of a dark brown solid with a yield of 93.5%.
[0043] (2) Synthesis of R1:
[0044] a: Dissolve 20.0mmol...
Embodiment 2
[0057] What this example prepares is the dye molecule described in general formula, R1 is where n=4, R2 is The reaction steps are as follows:
[0058] (1) Synthesis of p-iodophenylazide:
[0059] Dissolve 10.0mmol (2.19g) of p-iodoaniline in 6.5ml of hydrochloric acid aqueous solution (HCl: water = 1:1), put in a 100ml small beaker, stir at 0 degrees Celsius, and dissolve 10.0mmol (0.69g) of nitrous acid Sodium was dissolved in 35.4ml ice water, and was added dropwise in the reaction beaker, and 10.0mmol (0.65g) sodium azide was dissolved in 7.8ml ice water, and then added dropwise in the reaction vessel, and reacted for 30 minutes. The product was extracted with dichloromethane, and the organic layer was dried with 2.00 g of anhydrous magnesium sulfate. The magnesium sulfate was filtered out and then rotary evaporated. The product was placed in a vacuum drying oven and dried to obtain 2.25 g of a dark brown solid with a yield of 92%.
[0060] (2) Synthesis of p-aldehyde ...
Embodiment 3
[0070] What this example prepares is the dye molecule described in general formula, R1 is where n=4, R2 is For the reaction steps are as follows:
[0071] (1) Synthesis of p-iodophenylazide:
[0072] Dissolve 11.0mmol (2.41g) of p-iodoaniline in 7.2ml of hydrochloric acid aqueous solution (HCl: water = 1:1), place in a 100ml small beaker, stir at 0°C, and dissolve 11.0mmol (0.76g) of nitrous acid Sodium was dissolved in 35.4ml of ice water and added dropwise to the reaction beaker, 11.0mmol (0.72g) of sodium azide was dissolved in 8.6ml of ice water, then added dropwise to the reaction vessel and reacted for 30 minutes. The product was extracted with dichloromethane, and the organic layer was dried with 2.00 g of anhydrous magnesium sulfate. The magnesium sulfate was filtered out and then rotary evaporated. The product was placed in a vacuum drying oven and dried to obtain 2.39 g of a dark brown solid with a yield of 89%.
[0073] (2) Synthesis of R1:
[0074] a: Dissolve ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com