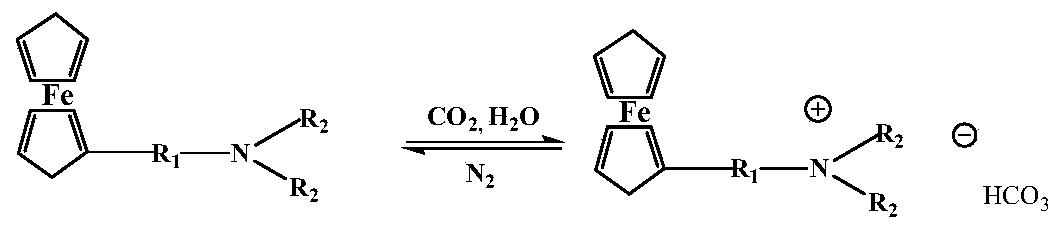
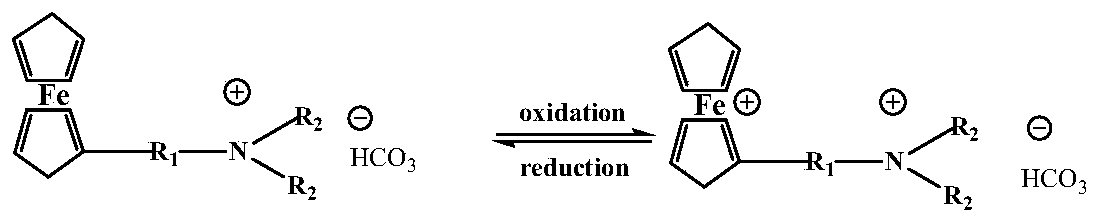
A ferrocene-based dual-stimuli-responsive surfactant
A dual-stimuli-responsive, surfactant technology, applied in the field of ferrocene-based dual-stimuli-responsive surfactants, can solve the problems of particle surface wettability regulation and lack of regulation of hydrophobic groups, and achieve excellent surface chemical properties, The synthesis method is simple and the effect of good surface properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: the synthesis of 12-alkyl tertiary amino ferrocene surfactant
[0031] First add 12.0539g of ferrocene and 19.0338g of anhydrous aluminum chloride into the three-necked flask, and add 50mL of dichloromethane as a solvent. Dissolve 16.7706g of 11-tertiary amine undecanoyl chloride in 30mL of dichloromethane, transfer it to a constant pressure funnel, and slowly add the 11-tertiary amine undecanoyl chloride solution dropwise to the three-necked flask. After the addition is complete, raise the reaction temperature to room temperature, react overnight, and remove the solvent after the reaction to obtain the acylated product. The acylated product was reduced with lithium aluminum hydride in ether solution. After 20 hours of reaction, the solvent was removed to obtain the reduced product. The reduced product was added to water, and carbon dioxide was introduced to obtain the surface of 12-alkyl tertiary aminoferrocene bicarbonate. active agent.
Embodiment 2
[0032] Example 2: 12-Alkyl Tertiary Amino Ferrocene Surfactant Foam
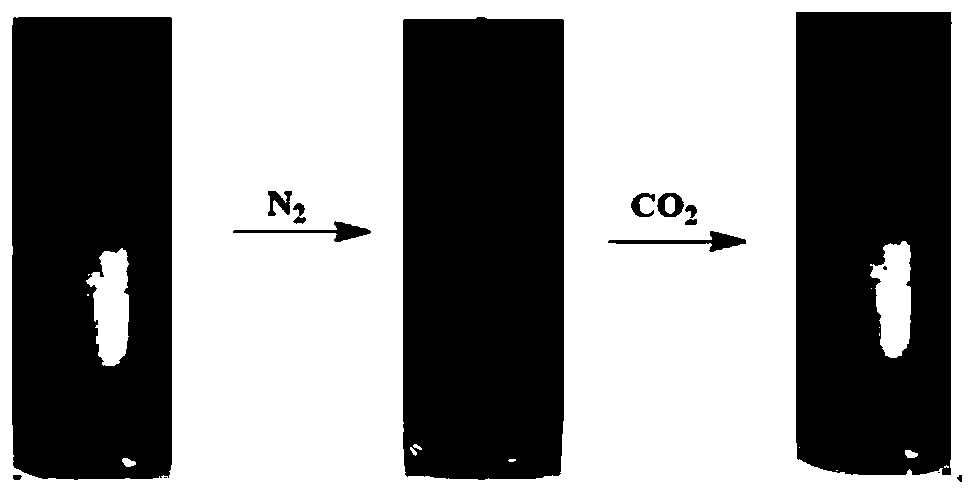
[0033] 12-Alkyl tertiary amino ferrocene surfactant is dissolved in water, and foam is obtained by shaking, as shown in the figure attached image 3 Introduce CO to the surfactant foam 2 and N 2 Subsequent changes are shown in Fig. Figure 4 Changes that occur when hydrogen peroxide is added to this surfactant foam.
Embodiment 3
[0034] Embodiment 3: 12-alkyl tertiary amino ferrocene surfactant emulsion
[0035] at 5cm 3 2×10 -3 In the 12-alkyl tertiary amino ferrocene surfactant aqueous solution of mol / L, add 5g n-decane, homogenize 2min under the condition of 8000rpm with high-speed homogenizer, obtain this surfactant emulsion, this emulsion The solution has carbon dioxide-redox dual stimulus response properties. Such as Figure 5 Introduce CO into the surfactant emulsion 2 and N 2 changes in appearance that occur after the Figure 6 Introduce CO into the surfactant emulsion 2 and N 2 Changes in post-microscope pictures.
[0036] Embodiment 2: Synthesis of 11-tertiary aminocarbonyl ferrocene surfactant
[0037] First add 12.0539g of ferrocene and 19.0338g of anhydrous zinc chloride into the three-necked flask, and add 50mL of dichloromethane as a solvent. Dissolve 16.7706g of 11-tertiary amine undecanoyl chloride in 30mL of dichloromethane, transfer it to a constant pressure funnel, and slo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com