Pregabalin sustained-release tablet medicinal composition and preparation method thereof
A technology of pregabalin and its composition, which is applied in the field of medicine, can solve the problems of uneven appearance of tablets, affecting the quality of sustained-release tablets, rough edges of tablets, etc., and achieves improved drug stability, simple composition, and simple and easy process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
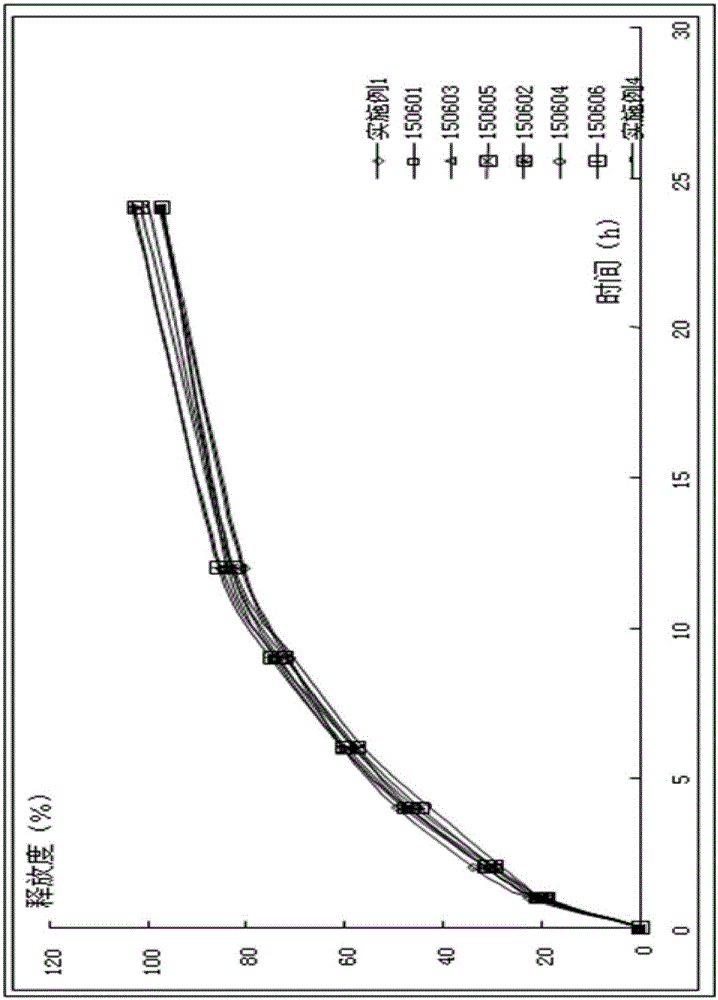
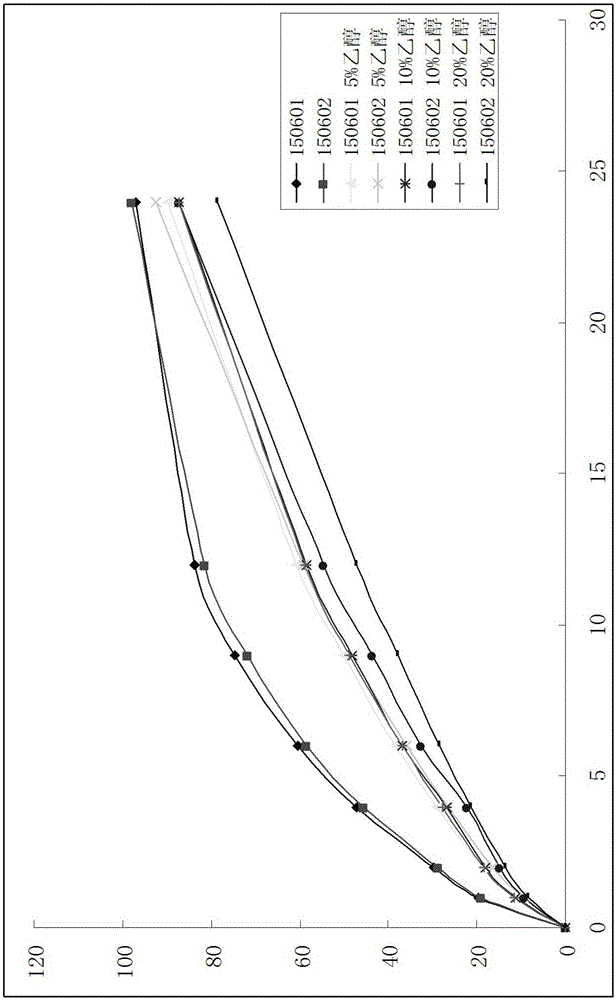
Image
Examples
Embodiment 1
[0039] Pregabalin Sustained Release Tablet Pharmaceutical Composition Prescription Composition: Pregabalin 1000g, Methylcellulose A4C 1350g, Methylcellulose SM-100 750g, Lactose 5500g, Mannitol 100SD 2000g, Silicon Dioxide 30g, Magnesium Stearate 30g, film coating powder (stomach-soluble type) 56g.
[0040]Preparation:
[0041] (1) Put methylcellulose A4C, methylcellulose SM-100, lactose, and pregabalin into a three-dimensional rocking mixer, control the speed at 10 rpm, and mix for about 20 minutes;
[0042] (2) Put silicon dioxide and magnesium stearate into the above-mentioned three-dimensional rocking mixer, control the rotating speed at 10 rpm, and continue mixing for about 5 minutes;
[0043] (3) Use ZP33 rotary tablet press, Φ8.5mm circular concave punching tablet;
[0044] (4) The film coating powder (stomach-soluble type) purchased from Shanghai Colorcon Coating Technology Co., Ltd. is added to the stirred 30% ethanol solution to form a film-coating powder (stomach-...
Embodiment 2
[0048] Prescription composition: Pregabalin 825g, hypromellose K100M 3000g, carbomer 971P 400g, mannitol 200SD2825g, microcrystalline cellulose PH102 2800g, magnesium stearate 150g.
[0049] Preparation:
[0050] (1) Mix 400g Carbomer 971P and 400g Hypromellose K100M in a mixer for about 3 minutes, and pass through a 18-20 mesh sieve;
[0051] (2) Put the mixed material of Carbomer 971P and hypromellose K100M, microcrystalline cellulose PH102, remaining hypromellose K100M, pregabalin, and mannitol 200SD into the three-dimensional shaking mixer, and control the speed at 10 rpm / min, mixing for about 20 minutes;
[0052] (3) Magnesium stearate is dropped into the above-mentioned three-dimensional rocking mixer, and the control speed is 10 rpm, and the mixture is continued for about 5 minutes;
[0053] (4) Tablets were compressed with a rotary tablet press, the die was a 20.5×10.5mm special-shaped punch, the tablet weight was controlled within ±4% of the theoretical tablet weig...
Embodiment 3
[0057] Prescription composition: Pregabalin 1650g, Hypromellose K100M 1500g, Hypromellose E50 1500g, Carbomer 971P 400g, Mannitol 200SD 2000g, Microcrystalline Cellulose PH102 2800g, Magnesium Stearate 150g.
[0058] Preparation:
[0059] (1) Mix 400g Carbomer 971P and 400g Hypromellose K100M in a mixer for about 3 minutes, and pass through a 18-20 mesh sieve;
[0060] (2) Put the mixture of carbomer 971P and hypromellose K100M, microcrystalline cellulose PH102, hypromellose E50, remaining hypromellose K100M, pregabalin, and mannitol 200SD into three-dimensional shaking and mixing In the machine, control the speed at 10 rpm, and mix for about 20 minutes;
[0061] (3) Magnesium stearate is dropped into the above-mentioned three-dimensional rocking mixer, and the control speed is 10 rpm, and the mixture is continued for about 5 minutes;
[0062] (4) Tablets were compressed with a rotary tablet press, the die was a 20.5×10.5mm special-shaped punch, the tablet weight was control...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com