Preparation and application of novel bitriamide organic compounds
An organic compound and bistriamide technology, applied in the field of radioactive waste treatment, can solve the problems of excessive concentration of extractant, difficulty in incineration, low distribution ratio, etc., and achieve significant selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
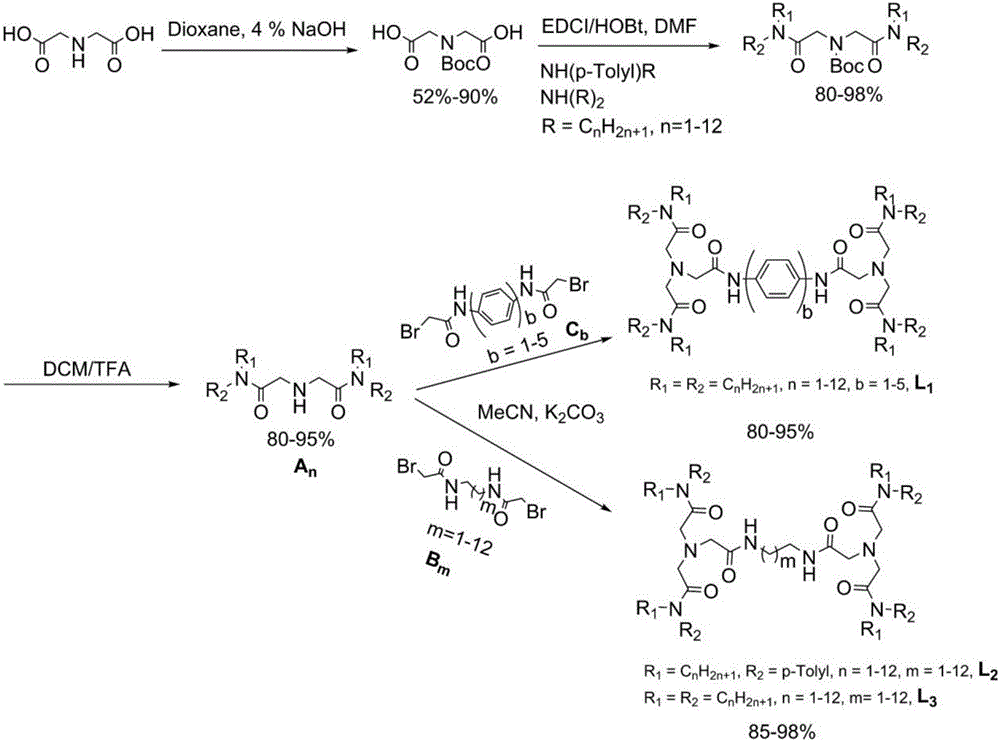
[0030] Embodiment 1 Novel amides organic compound L 3 class extractant ( figure 1 ), L 3-58 Prepare as follows:
[0031]
[0032] Using iminodiacetic acid as the initial raw material, iminodiacetic acid was dissolved in 4% NaOH aqueous solution and dioxane as the solvent, reacted for 72 h, and passed through di-tert-butyl dicarbonate ((Boc) 2 ) to protect the imino group, through recrystallization under the conditions of ethyl acetate and n-hexane, volume ratio (1:2), to obtain pure diacetic acid containing tert-butoxycarbonyl (Boc) protection. Then, the diacetic acid that obtains Boc protection takes N,N-dimethylformamide (DMF) as solvent again, with 1-(3-dimethylaminopropyl group)-3-ethylcarbodiimide hydrochloride ( EDCI), 1-hydroxybenzotriazole (HOBt) as a catalyst, under the protection of nitrogen, react at 0-25 ℃ for 24 h, add di-n-octylamine, and carry out amidation reaction. After the reaction was complete, the product was extracted by adding ethyl acetate, washe...
Embodiment 2
[0048] Embodiment 2 Novel amides organic compound L 1 class extractant ( figure 1 ), L 1-14 Prepare as follows:
[0049]
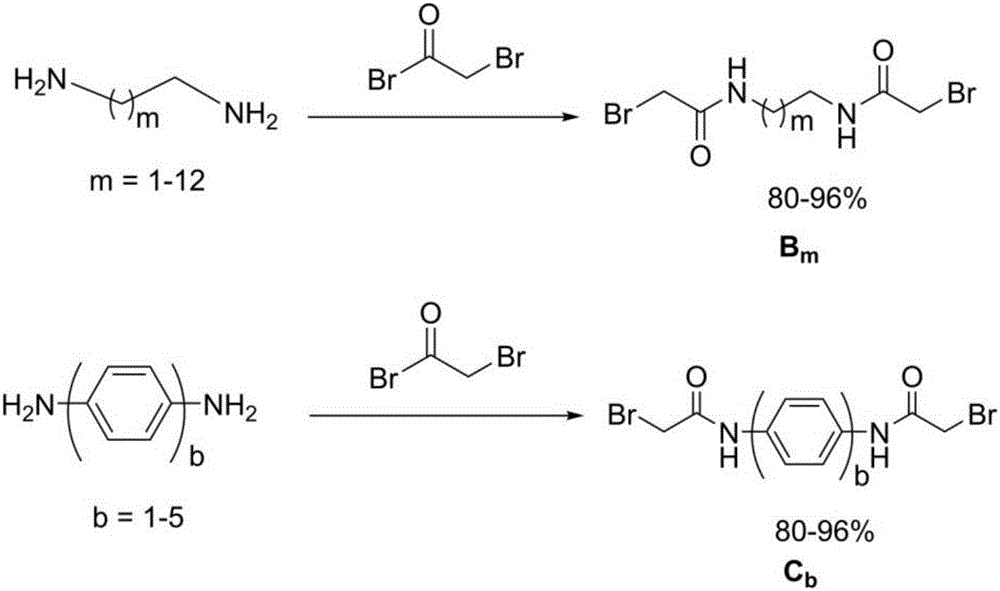
[0050] Synthetic steps such as figure 2 Shown, wherein, intermediate secondary amine A 4 The preparation is the same as in Example 1, and the dibromide C 1 The preparation is by taking bromoacetyl bromide, p-phenylenediamine as raw material ( figure 2 ), using tetrahydrofuran (THF) as a solvent, reacting at 0-25 °C for 2 h, and then washing the product several times with water to obtain a solid product.
[0051] Finally, put A 4 and C 1 Under the condition of acetonitrile as solvent, react at 40 - 85 ℃ for 5 h. After the reaction is complete, remove the solvent, add ethyl acetate to extract the product, successively pass through 5% aqueous hydrochloric acid solution, saturated sodium bicarbonate solution, and saturated saline solution to wash three times, dry with anhydrous sodium sulfate, remove the solvent, and dry at 60 °C to obtain light y...
Embodiment 3
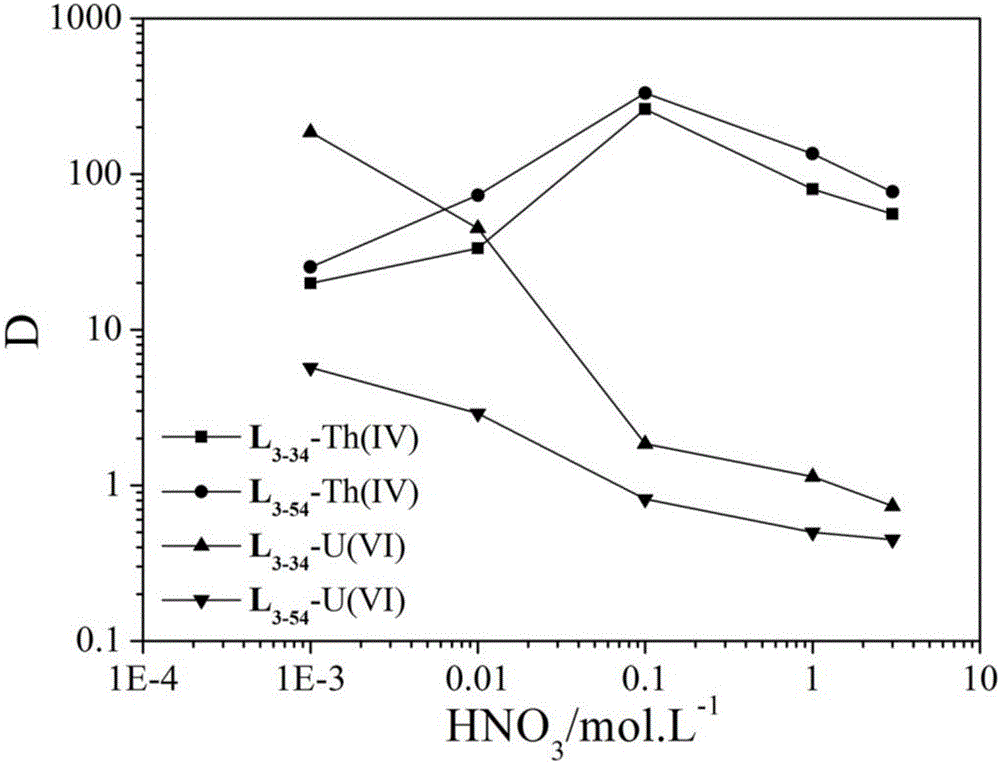
[0056] Embodiment 3 Novel amides organic compound L 3-14 Used in liquid-liquid extraction experiments of Th(IV) and U(VI).
[0057] The specific method is as follows:
[0058] Prepared in toluene with a concentration of 0.01 mol.L -1 the L 3-14 As the organic phase; and respectively in different concentrations of nitric acid system (1.0 10 -3 - 10 mol.L -1 HNO 3 ) is prepared to be about 50 mg.L -1 Thorium nitrate aqueous solution and uranyl nitrate aqueous solution are used as the aqueous phase. Take the same volume (1 - 2 mL) of the organic phase and the aqueous phase, mix them, and stir at 25 °C. After about 1 hour, the extraction reaches equilibrium. Then let stand to separate the organic phase and the aqueous phase, centrifuge, and then test the contents of metal thorium ions and uranyl ions in the aqueous phase by ICP-AES, and finally calculate the content of the same metal ion in the organic phase and the aqueous phase. The ratio is the distribution ratio D...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com