Aggregation-induced emission probe based on quinazolinones compound as well as preparation method and application of aggregation-induced emission probe
A technology for quinazolinone and compound, which is applied in the application field of quinazolinone compound and ammonia gas detection, and can solve the problems of difficult synthesis, complex compound structure, inconvenience of making it portable, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] According to the synthetic route of the present invention, synthesize the compound of formula II with the following specific structure:
[0117] (1) Synthesis of Compound II-1: 2-[2-hydroxyphenyl]-4-(3H)-quinazolone (R 1 = H, R 2 =H)
[0118]
[0119] Anthranilamide (272mg, 2mmol), salicylaldehyde (244mg, 2mmol) and iodine (508mg, 2mmol) were placed in 15mL of ethanol and reacted under reflux for 6 hours. After the reaction was completed, 5% by mass fraction of sodium thiosulfate solution was added to remove unreacted iodine. A large amount of white precipitate precipitated out of the reaction solution, which was filtered, washed with water (10 mL×2) and ethanol (10 mL×2), and dried in vacuo to obtain a white solid with a mass of 390 mg (yield 82%).
[0120] 1 H NMR (d 6 -DMSO,500MHz):δ13.78(br s,1H),12.48(br s,1H),8.23(dd,J 1 =8.0Hz,J 2 =1.5Hz,1H),8.16(dd,J 1 =8.0Hz,J 2 =1.5Hz,1H),7.86(td,J 1 =8.5Hz,J 2 =1.5Hz, 1H), 7.77(d, J=8.0Hz, 2H), 7.56(td, J 1 =8....
Embodiment 2
[0138] According to the synthetic route of the present invention, the formula I compound of following specific structure is synthesized on the basis of the compound of Example 1:
[0139] (1) Synthetic (R 1 = H, R 2 = H, R 3 = methyl)
[0140]
[0141] 2-[2-Hydroxyphenyl]-4-(3H)-quinazolinone (280mg, 1.0mmol) was added to 5mL methanol solution dissolved with sodium methoxide (108mg, 2.0mmol), and reacted at room temperature under nitrogen protection 5 minutes. After the reaction solution became clear, methanol was removed by rotary evaporation. Subsequently, 10 mL of THF and acetic anhydride (408 mg, 4.0 mmol) were added and reacted at room temperature for 2 hours under nitrogen protection. After the reaction was completed, THF was removed by rotary evaporation, the residue was washed with water, and then dried in vacuo to obtain a white solid with a mass of 266 mg (yield: 95%).
[0142] 1 H NMR (CDCl 3 ,500MHz):δ10.42(br s,1H),8.29(dd,J 1 =7.5Hz,J 2 =1.0Hz,1H),8....
Embodiment 3
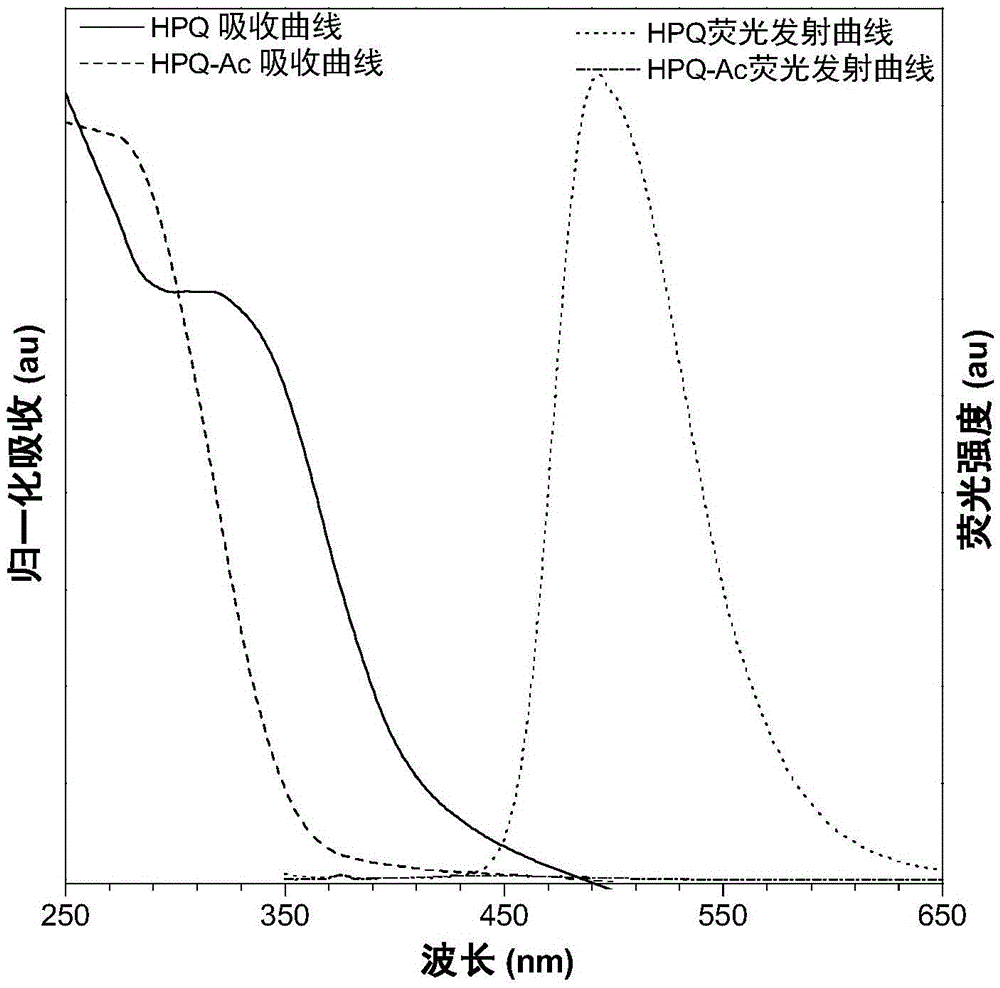
[0150] Fluorescence property test of formula I compound and formula II compound
[0151] 1. Fluorescence quantum yield is measured by C11347 Quantaurus_QY instrument.
[0152] 2. Research method of fluorescence emission spectrum in tetrahydrofuran / water:
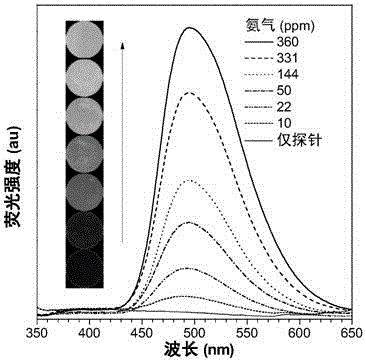
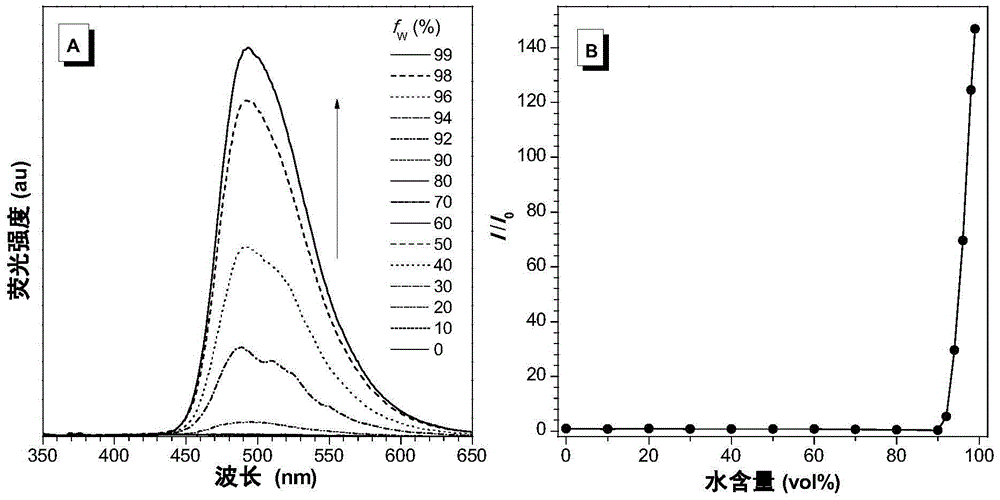
[0153] tetrahydrofuran and water according to different volume ratios (THF: water = 100:0, 90:10, 80:20, 70:30, 60:40, 50:50, 40:60, 30:70, 20:80, 10:90, 1:99) to form mixed solutions with different water contents, compound I-1 and compounds II-1~II-5 were dissolved in these mixed solutions respectively, so that the concentration of the compound was 10 -5 mol L -1 , using appropriate excitation light to detect the fluorescence emission of compound I-1 and compounds II-1-II-5 in these solvent systems.
[0154] see attached results Figure 1-6 . When the water content in the mixed solution system is continuously increased, the solubility of compounds II-1-II-5 gradually decreases and thus aggregates, and the fluorescence ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com