Fusion protein used for rapidly detecting type I diabetes
A fusion protein, fusion protein technology, applied in fusion polypeptides, microorganism-based methods, hybrid peptides, etc., can solve the problems of low efficiency, insufficient specificity, single assay, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
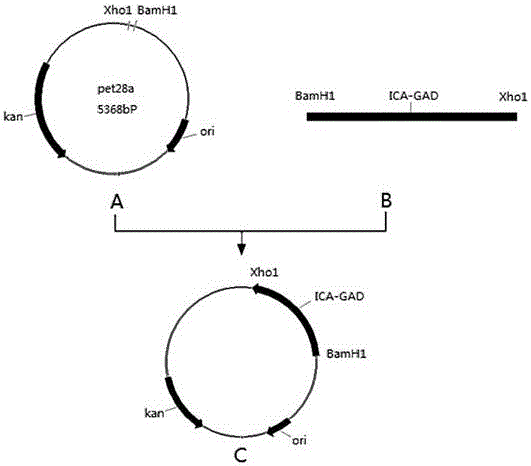
[0017] Example 1: Fusion and expression of genes
[0018] (1) The target DNA sequence is artificially synthesized.
[0019] (2) PCR amplification of the target gene: In order to ensure the fusion expression of the two, Primer5.0 was used to design specific amplification primers according to the sequence of the target gene. The primers were designed with BamH I and Xho I as restriction sites. The primer sequences are:
[0020] cgggatccat ggcatctccg ggctctggct tttggtct SEQ ID NO: 1
[0021] ccctcgagtc atgcattgag caattcgtgg ttc SEQ ID NO: 2
[0022] A high-fidelity DNA amplification system was used to perform PCR cycles using the synthesized DNA sequence as a template to obtain products.
[0023] (3) After amplification, the target gene fragments were recovered according to the AxyPrep DNA Gel Recovery Kit. Insert the target gene into the vector: connect the cDNA product to the vector, transform it into the PET-28a vector, screen according to the marker of the recombinant vec...
Embodiment 2
[0025] Example 2: Protein Purification
[0026] The induced bacteria containing the target protein were collected by centrifugation, ultrasonically disrupted, and the supernatant was collected by centrifugation. In order to improve the purification purity of the target protein, we improved the traditional method of purifying his fusion protein by adding β-mercaptoethanol (to make the final concentration 5mM) in the E. coli supernatant after induction of expression. Then use the imidazole buffer containing 300mM Nacl, 20mM Tris-Hcl and 40mM to bind the target protein to the HIS Trap FF affinity column, and then use the imidazole buffer containing 300mM Nacl, 20mM Tris-Hcl and 500mM to elute the target protein. It was dialyzed overnight at 4°C in PBS buffer, and the protein concentration was determined to be 1 mg / ml by BCA method.
Embodiment 3
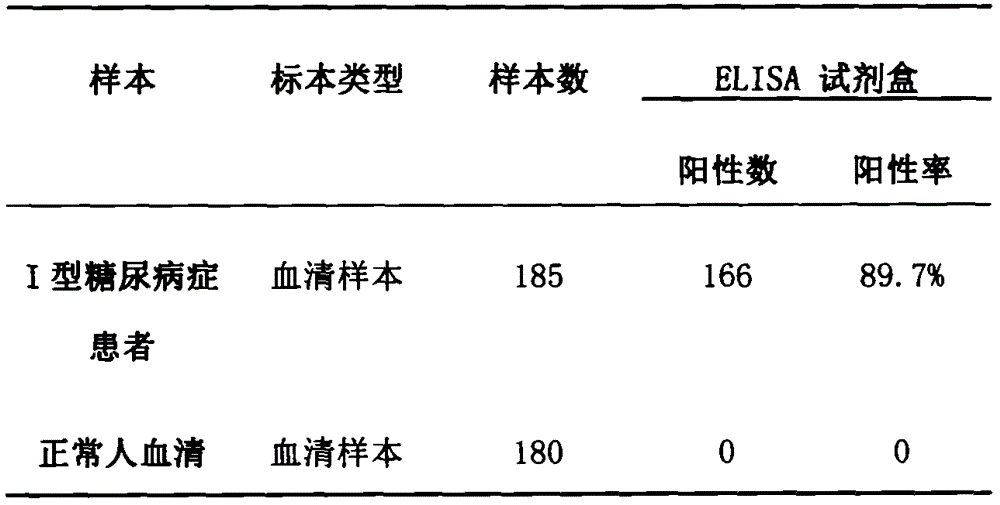
[0027] Example 3: Immunological activity was determined by Elisa detection.
[0028] 1. Coating: Dilute the fusion protein ICA-GAD with 0.05M PH9, carbonate coating buffer to a protein content of 1-10 μg / ml, add 0.1ml to the reaction well of each polystyrene plate, 4 ℃ overnight. The next day, the solution in the well was discarded, and washed 3 times with washing buffer, 3 minutes each time.
[0029] 2. Adding samples: Add 0.1ml of a certain dilution of the sample to be tested to the above-mentioned coated reaction wells, and incubate at 37°C for 1 hour. Then wash. (Make blank wells, negative control wells and positive control wells at the same time).
[0030] 3. Add enzyme-labeled antibody: Add 0.1ml of freshly diluted enzyme-labeled antibody (diluted after titration) to each reaction well, incubate at 37°C for 0.5-1 hour, and wash.
[0031] 4. Add substrate solution for color development: add 0.1 ml of temporarily prepared TMB (tetramethylbenzidine) substrate solution t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com