Preparation method of waterborne polyurethane with hyperbranched structure silicone oil side chain
A technology of hyperbranched silicone oil and water-based polyurethane, which is applied in the field of fine chemicals and achieves the effects of easy control of operating conditions, good market prospects, and reduced water absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
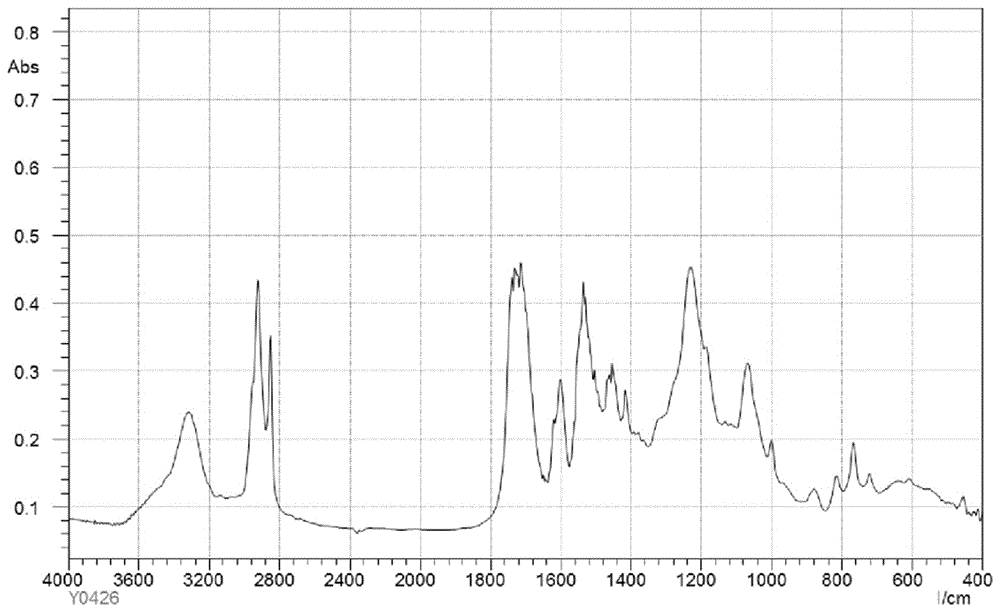
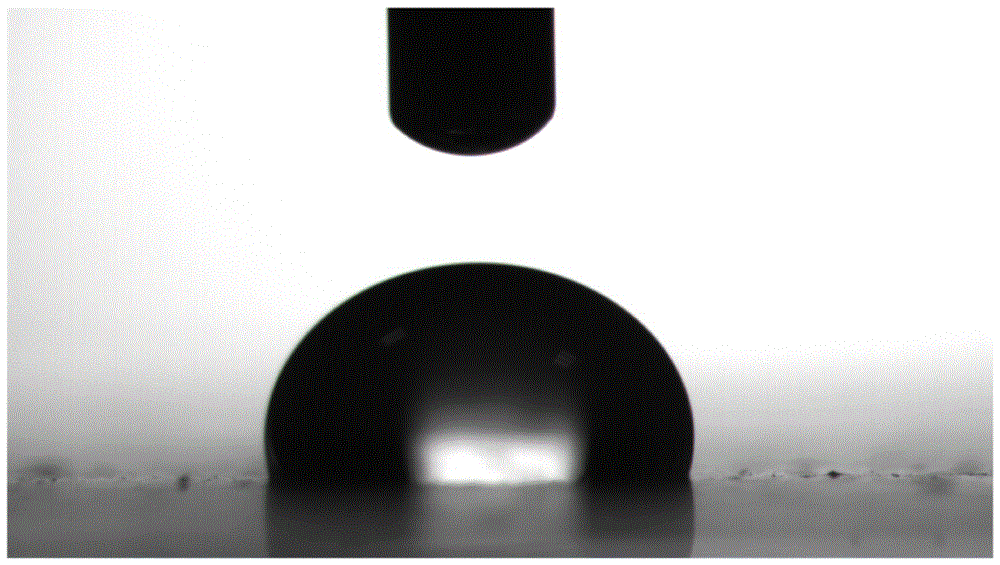
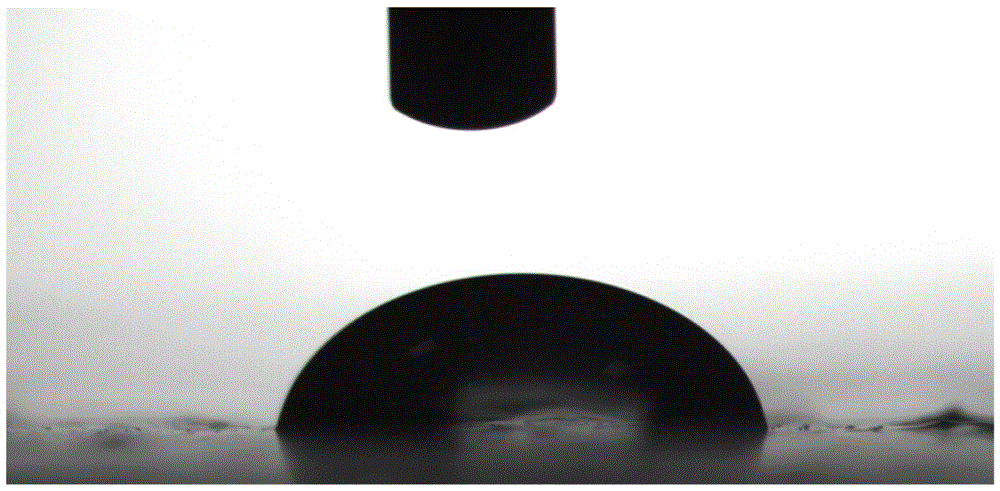
Image
Examples
preparation example Construction
[0035] The preparation method of described containing hyperbranched silicone oil side chain modified aqueous polyurethane emulsion, concrete steps are as follows:
[0036] 1) Stir and react 100 parts of polyether polyol, 10-50 parts of hyperbranched silicone oil and 20-80 parts of diisocyanate at 65-100°C for 1-3 hours under catalyst conditions to obtain isocyanate-terminated polyurethane prepolymer;
[0037] in,
[0038]In step (1), the polyether polyol is selected from one or a combination of not less than two of polypropylene glycol or polytetrahydrofuran ether glycol, and has a molecular weight of 300-6000;
[0039] In step (1), the hyperbranched amino silicone oil has a general structural formula as shown in the following formula (I):
[0040]
[0041] Formula (Ⅰ)
[0042] Wherein, in the formula (I), X, Y, and Z are natural numbers of 10 to 100 respectively;
[0043] In step (1), the diisocyanate is selected from isophorone diisocyanate (IPDI), hexamethylene diisoc...
Embodiment 1
[0058] Add 100 g of PPG1000 and 50 g of hyperbranched amino silicone oil (Momentive, SF1921) into a three-necked flask equipped with a thermometer and a reflux condenser. Under stirring conditions, add 36 g of toluene diisocyanate and 0.25 g of dibutyltin dilaurate, Stir and react at 65°C for 3 hours to obtain an isocyanate-terminated polyurethane prepolymer; then, add 5g of trimethylolpropane chain extender and 5g of N-methyldiethanolamine chain extender to the above polyurethane prepolymer, and use acetone Dilute with 50g, stir and react at 45°C for 6 hours, analyze the residual NCO content by the di-n-butylamine method, wait until the calculated value is reached, then cool down to room temperature (below 30°C), then add dimethyl sulfate diluted with 2.8g acetone Esters (5.5g) were quaternized, and the reaction was maintained at room temperature for 1 hour to obtain a quaternized cationic isocyanate-terminated polyurethane prepolymer; the above-mentioned quaternized cationic ...
Embodiment 2
[0061] Add 45 g of PPG300, 55Gppg6000 and 10 g of hyperbranched amino silicone oil (Momentive, SF1708) into a three-necked flask equipped with a thermometer and a reflux condenser. Under stirring conditions, add 80 g of isophorone diisocyanate and dibutyltin dilaurate Ester 1g, stirred and reacted at 100°C for 1 hour to obtain an isocyanate-terminated polyurethane prepolymer; then, add 1g of butanediol chain extender and 20g of N-phenyldiethanolamine chain extender to the above polyurethane prepolymer, Dilute with 20g of propyl acetate, stir and react at 80°C for 2 hours, analyze the residual NCO content by the di-n-butylamine method, wait until the calculated value is reached, then cool down to room temperature (below 30°C), then add 46g of propyl acetate Diluted methyl iodide (23g) was quaternized, and the reaction was kept at room temperature for 3 hours to obtain a quaternized cationic isocyanate-terminated polyurethane prepolymer; the above-mentioned quaternized cationic p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| contact angle | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com