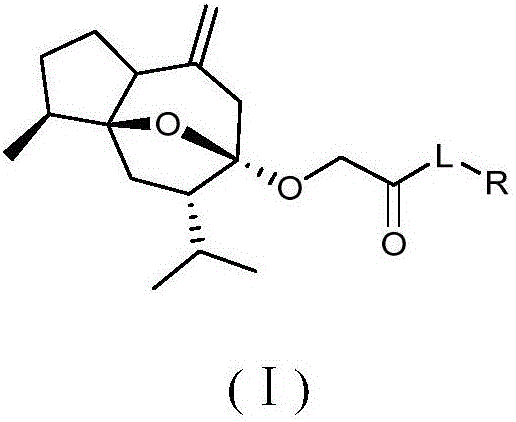
Curcumol derivatives with anti-tumor activity, and preparation method and application of curcumol derivatives
A technology of anti-tumor activity and curcumol, applied in the field of medicine, can solve the problems of limited application and clinical, low curcumol, enhanced immunogenicity, etc., to facilitate the development and clinical application of preparations, enhance water solubility, and improve bioavailability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the preparation of curcumol derivative
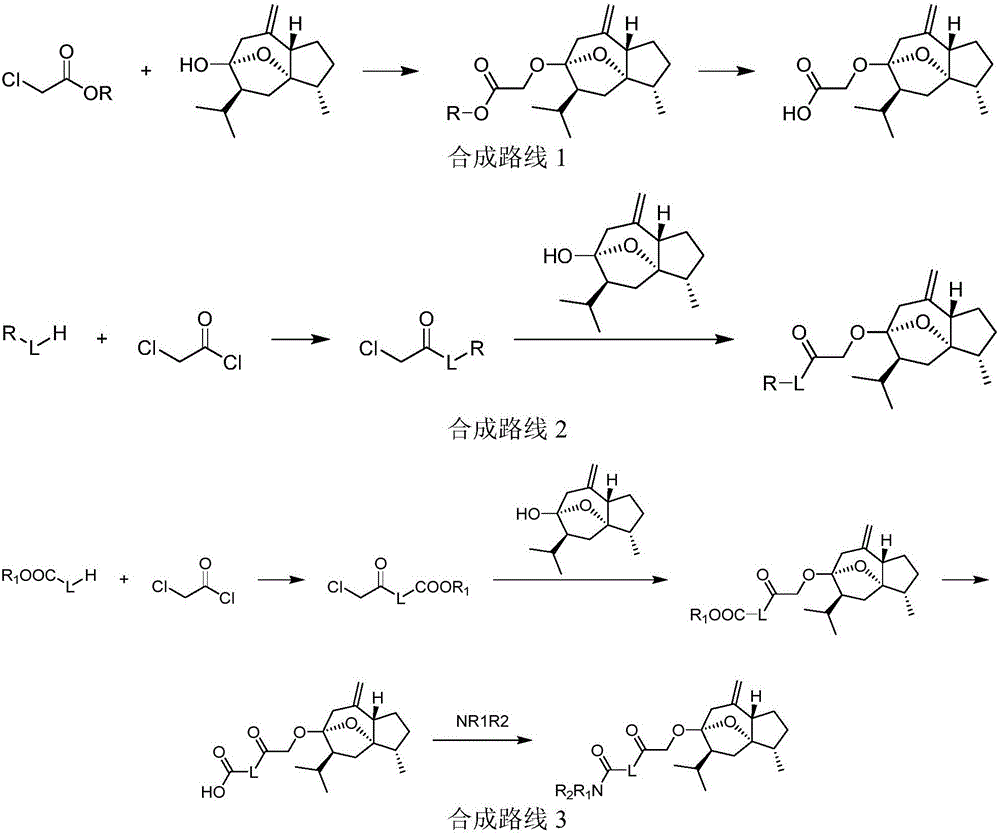
[0029] (1) Preparation scheme 1
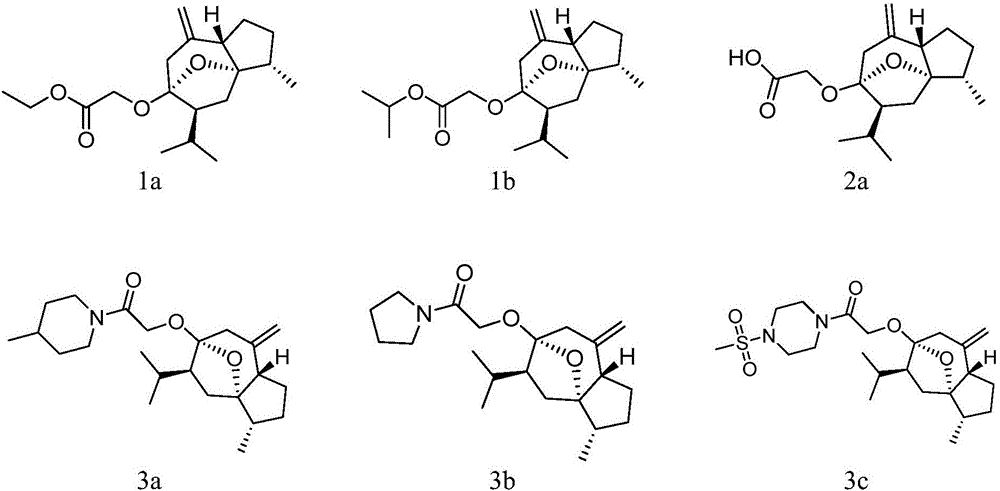
[0030] Compound 1a Compound 2a For example, the synthetic route 1 is as follows:
[0031]
preparation approach 1
[0033] (1) Take 4.72g of curcumol, dissolve it in 80mL of dry tetrahydrofuran, add 1.2g (60%) of sodium hydride, heat to reflux for 2h, cool, add 2.93g of ethyl chloroacetate, and continue to reflux for 14h. After the reaction is complete, concentrate, add 100 mL of water, extract 3 times with ethyl acetate, combine the ethyl acetate layers, backwash the ethyl acetate layer with saturated sodium chloride aqueous solution, dry over anhydrous magnesium sulfate, and concentrate under reduced pressure to obtain a brown oily product , a colorless oily product 1a was obtained by silica gel column chromatography.
[0034] (2) Take 1.61g of 1a, dissolve it in a mixed solution of 24mL ethanol and 8mL water, add 0.63g of lithium hydroxide monohydrate, and stir at room temperature for 4h. Remove ethanol by rotary evaporation, add appropriate amount of water, adjust pH to 5-6 with 10% hydrochloric acid, extract 3 times with ethyl acetate, combine ethyl acetate layer, backwash ethyl acetat...
preparation approach 2
[0039] (1) Take 2.48g of 4-methylpiperidine, dissolve it in 20mL of dry tetrahydrofuran solution, add 7.59g of triethylamine, and slowly add 10mL of tetrahydrofuran containing 4.24g of chloroacetyl chloride dropwise under stirring at room temperature, for 1h After the dropwise addition, continue to react for 2 h at room temperature. After the reaction is complete, concentrate, add 40 mL of water, extract 3 times with ethyl acetate, combine the ethyl acetate layers, backwash the ethyl acetate layer with a saturated sodium chloride aqueous solution, and anhydrous Dried over magnesium sulfate, concentrated under reduced pressure to obtain a tan crude product, dried in a vacuum oven, and directly put into the next reaction.
[0040] (2) Take 1.18 g of curcumol, dissolve it in 20 mL of dry tetrahydrofuran solution, add 0.3 g of sodium hydride, heat and reflux for 2 hours, let it cool slightly, add 1.05 g of the compound obtained in the previous step (1), and continue heating and ref...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com