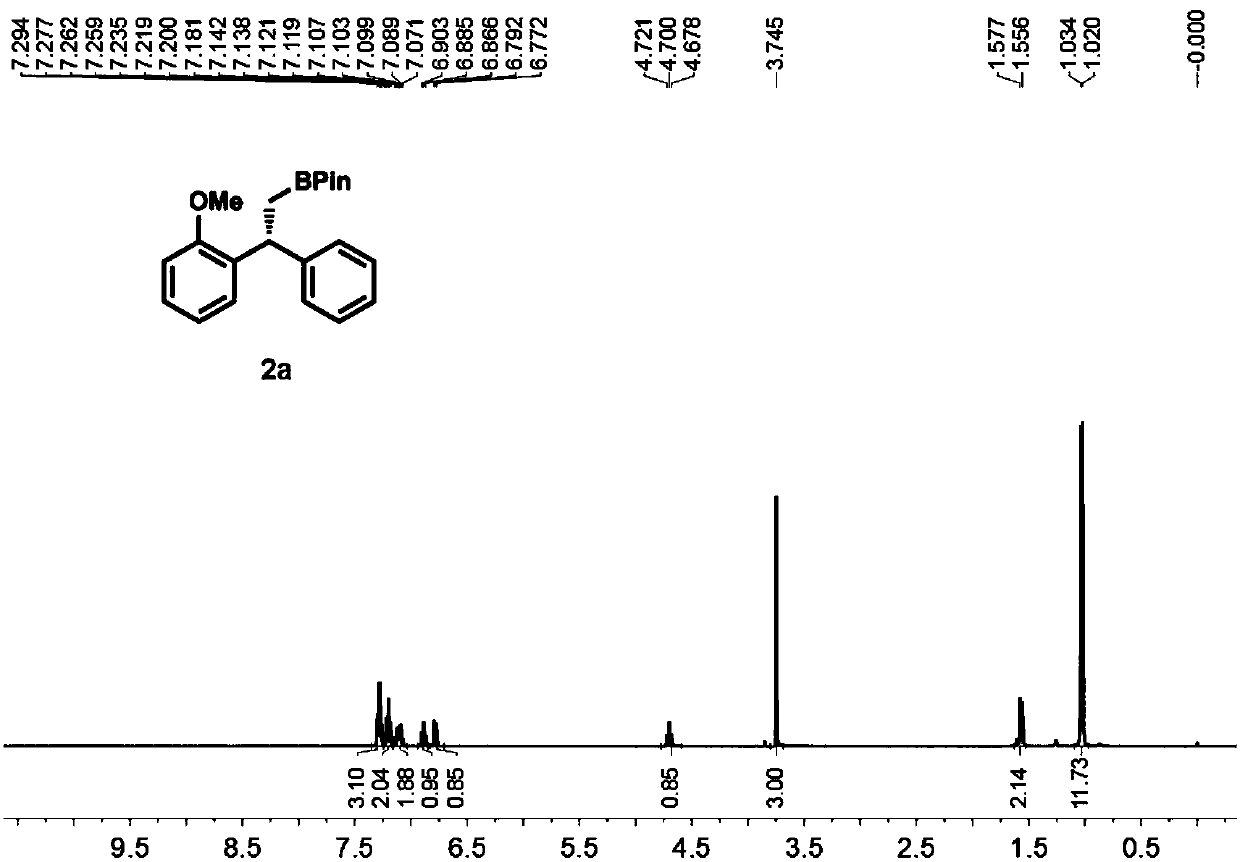
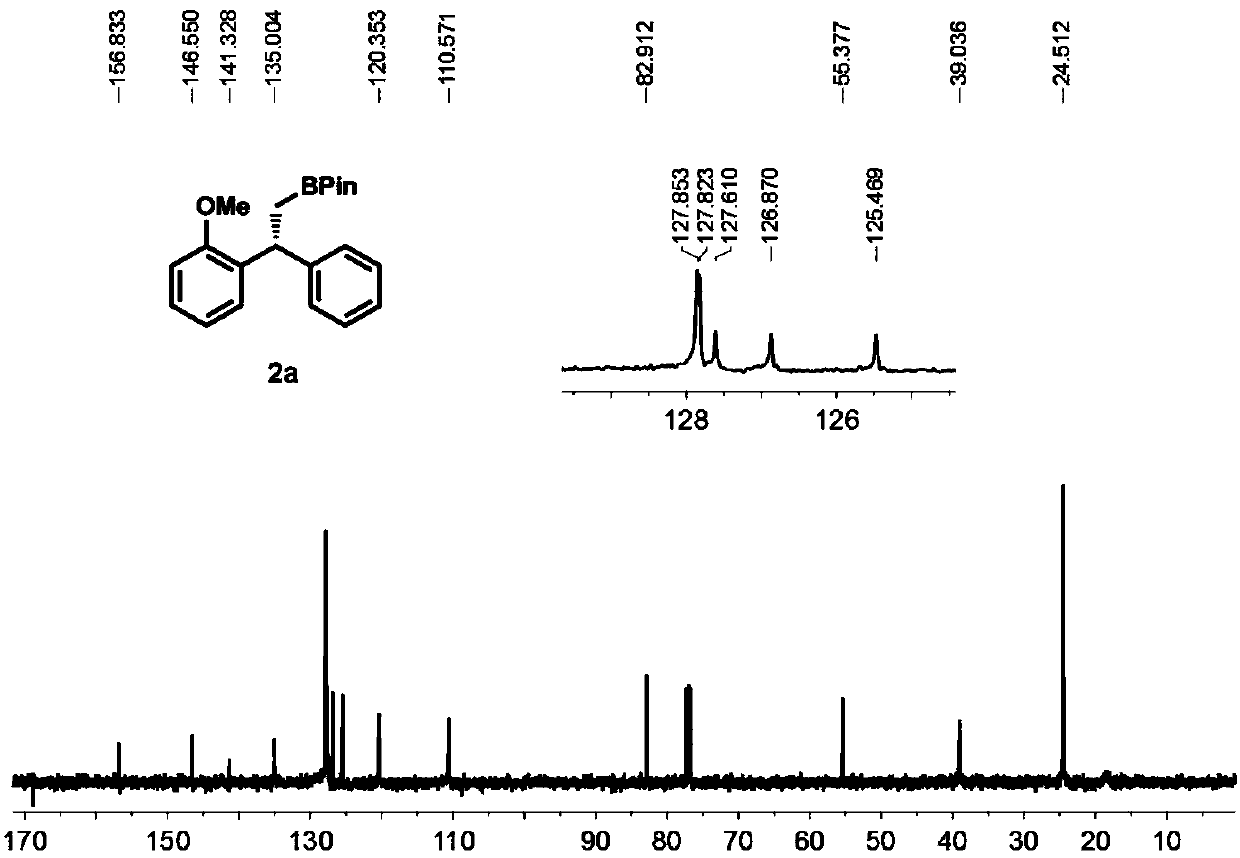
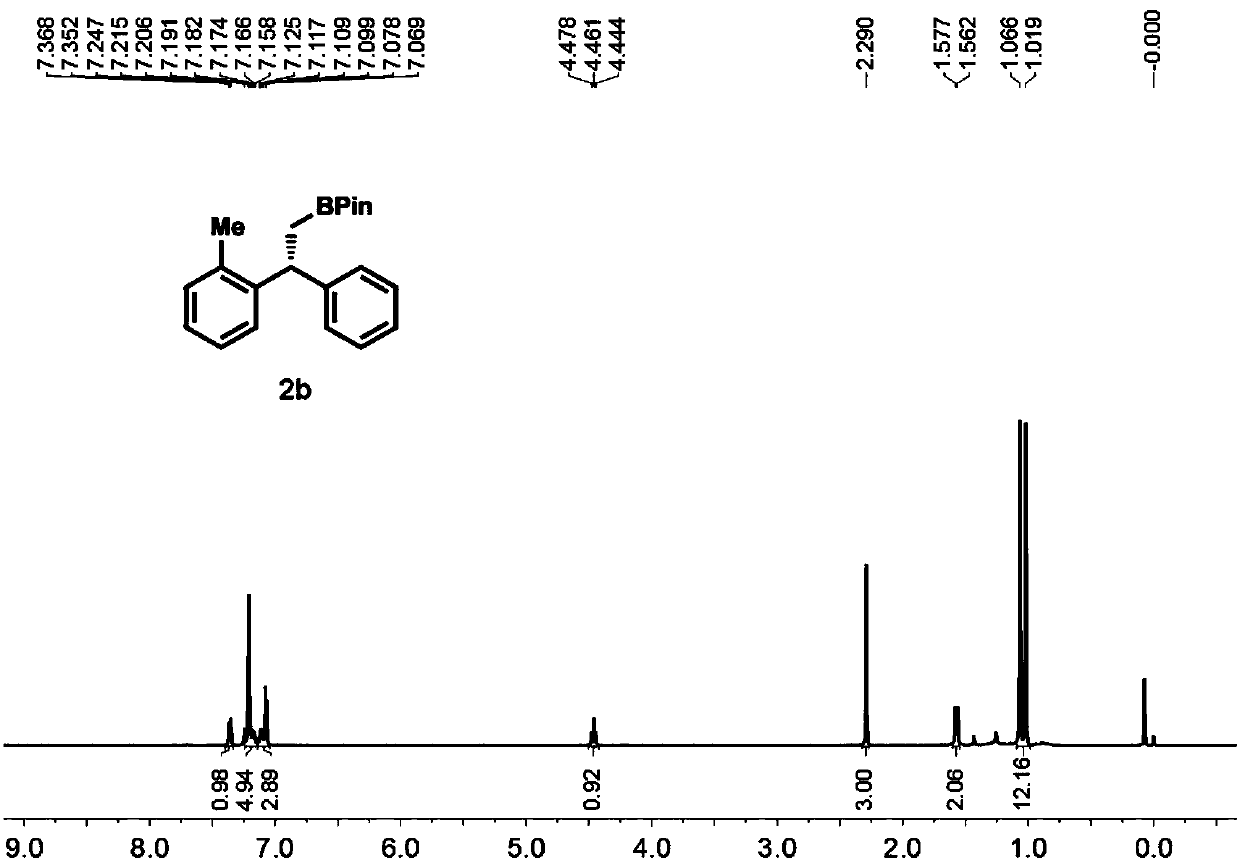
High-enantioselectivity 1,1-diarylboroalkanes and preparation method thereof
A technology for diarylborated alkanes and compounds, which is applied in the field of high enantioselective 1,1-diarylborated alkanes and their preparations, and can solve problems that have not been reported in the literature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] The present invention also provides a method for preparing high enantioselectivity 1,1-diarylborated alkane compounds, the method comprising:
[0037] Step 1: reacting diaryl ketone, Wittig reagent and n-butyllithium in a polar solvent to obtain 1,1-diarylethene compound;
[0038] Step 2: Add catalyst, ligand ((2S,4S)-(-)-2,4-bis(diphenylphosphonium)pentane), counter ion and alkali to the reaction device, and then add the obtained 1,1-Diarylethene compound reacts with diboronic acid pinacol ester to obtain 1,1-diarylborated alkanes with high enantioselectivity.
[0039] According to the present invention, add Wittig reagent and polar solvent in the reaction device, preferably lower the temperature of the reaction device to -78°C, then add n-butyllithium dropwise, after the dropwise addition, preferably react at room temperature for 30min, then in the reaction device Add diaryl ketone to the mixture, preferably react at room temperature for 24 hours, TLC detects that th...
Embodiment 1
[0047] 1) Preparation of 1,1-diarylethene 1a
[0048]
[0049] To a 25 mL round bottom flask equipped with a magnetic stirrer was added methyltriphenylphosphine bromide (2.6 mmol) and tetrahydrofuran (5 mL). Move the reaction to -78°C, add n-butyllithium (2.6mmol) dropwise to the system, after the dropwise addition, react at room temperature for half an hour, then add diaryl ketone (2mmol) to the system, stir at room temperature for 24h, TLC The detection substrate disappears and the reaction ends. Pour saturated ammonium chloride aqueous solution (10mL) into the reaction, extract with dichloromethane (3×10mL), combine the organic phases, dry over anhydrous sodium sulfate, filter with suction, then distill under reduced pressure to remove the organic solvent, pass through a silica gel column layer Analysis (eluent is V 石油醚 :V 乙醚 =20:1), a colorless oily liquid was obtained, the structure of the product was confirmed to be 1a by NMR and MS, and the yield was 90%.
[0050...
Embodiment 2
[0057] 1) Preparation of 1,1-diarylethene 1b
[0058]
[0059] Under nitrogen protection, methyltriphenylphosphine bromide (2.6 mmol) and tetrahydrofuran (5 ml) were added to a 25 mL round bottom flask equipped with a magnetic stirring device. Move the reaction to -78°C, add n-butyllithium (2.6mmol) dropwise to the system, after the dropwise addition, react at room temperature for half an hour, then add diaryl ketone (2mmol) to the system, stir at room temperature for 24h, TLC The detection substrate disappears and the reaction ends. Pour saturated ammonium chloride aqueous solution (10mL) into the reaction, extract with dichloromethane (3×10mL), combine the organic phases, dry over anhydrous sodium sulfate, filter with suction, then distill under reduced pressure to remove the organic solvent, pass through a silica gel column layer Analysis (eluent is petroleum ether) to obtain a colorless oily liquid, the structure of the product was confirmed to be 1b by NMR and MS, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com