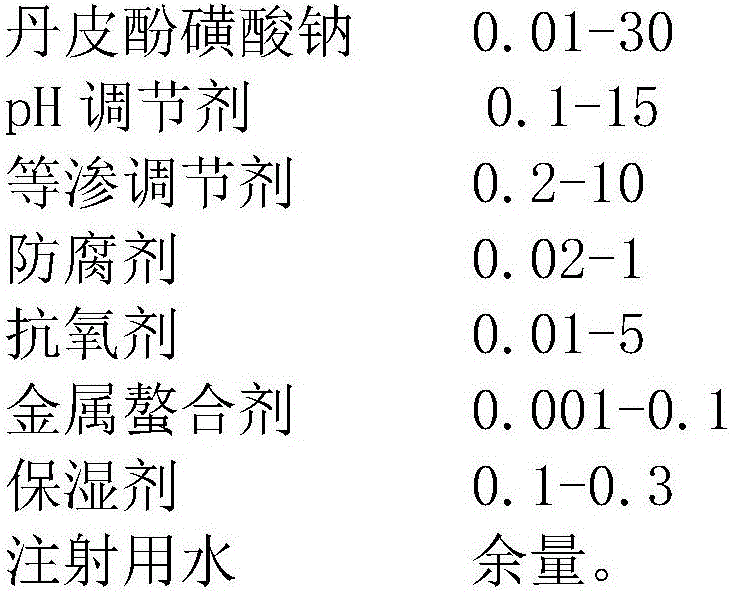
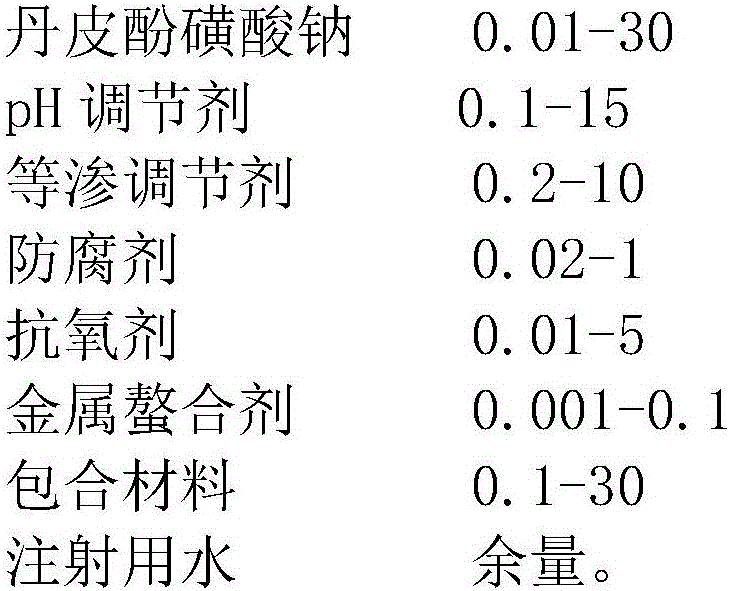
Sodium paeonolsilate ophthalmic preparation
A technology of sodium paeonol sulfonate and ophthalmic preparations, applied in the field of sodium paeonol sulfonate ophthalmic preparations, can solve problems such as application and reports of unseen eye preparations, and achieves improved ocular bioavailability and improved ocular bioavailability. Stability, the effect of reducing toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: Sodium Paeonol Sulfonate Eye Drops
[0044] Take 50mL of water for injection, add NaCl 0.7g, boric acid 1.9g, sodium bisulfite 0.2g, EDTA-2Na 0.03g, benzalkonium chloride 0.03g, sodium hyaluronate 0.1g, stir to dissolve, then add paeonol Sodium sulfonate 3.5g, after dissolving completely, add water for injection to 100mL, adjust pH to 6.0 with citric acid, filter with 0.22μm microporous membrane, aseptically dispense into brown plastic eye drop bottles, and pack to get ready.
Embodiment 2
[0045] Embodiment 2: Paeonol Sulfonate Sodium Liposome Eye Drops
[0046] Using the film dispersion method, take 1g of soybean lecithin and 0.75g of cholesterol dissolved in chloroform as the oil phase, use a rotary evaporator to evaporate to dryness at 55°C to form a film, remove the chloroform, stop the rotation and keep vacuuming for 2 hours, then stop, add sodium sulfite 0.15g, 0.03g of EDTA-2Na, 0.02g of benzalkonium bromide and 0.2g of paeonol sodium sulfonate in 50mL of phosphate buffer (pH6.0), shake vigorously for 40min, wash the membrane at 4℃ Homogenize with a high-pressure homogenizer (homogenization pressure 80Mpa, cycle times 5 times) to obtain paeonol sulfonate liposome eye drops. Slowly drop the prepared paeonol sulfonate liposome eye drops into 50ml of phosphate buffer (1%, w / v) of chitosan oligosaccharide (50kDa), stir and mix at 10°C for 60min to obtain Chitooligosaccharide-coated paeonol sulfonate liposome suspension was adjusted to isotonicity with NaCl, ...
Embodiment 3
[0047] Embodiment 3: Paeonol Sulfonate Sodium Inclusion Complex Eye Drops
[0048]Take 1g of HP-β-cyclodextrin, add 75mL of water for injection, heat to dissolve, add 0.1g of sodium paeonol sulfonate, stir to dissolve, put it on an ultrasonic oscillator for 10min, then add 0.6g of sodium chloride, boric acid 0.4g, 1.3g of borax, 0.1g of methylparaben, 0.02g of propylparaben, 0.04g of EDTA-2K, 0.1g of sodium bisulfite, stir to dissolve, and adjust the pH to 6.5 with HCl. 0.22μm micropore Membrane filtration, aseptically dispensed into brown plastic eye droppers, ready to pack.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com