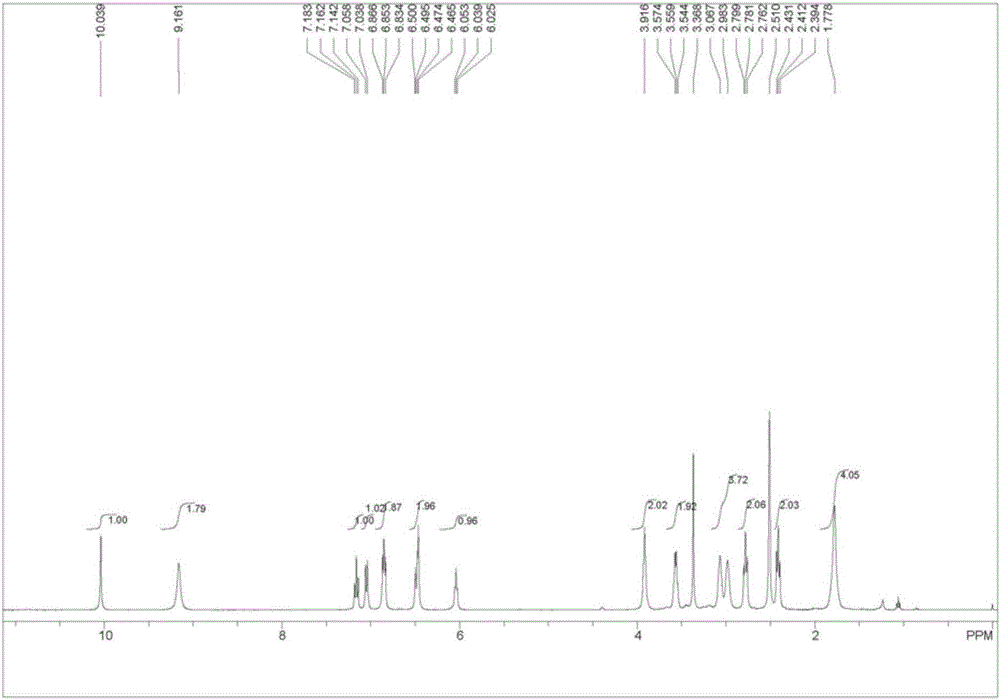
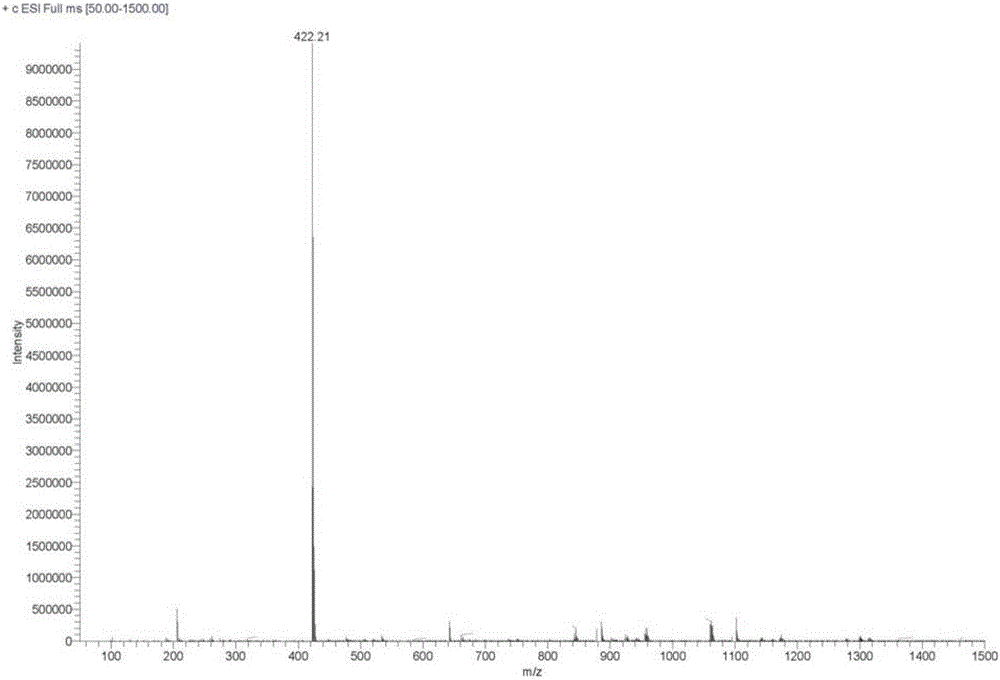
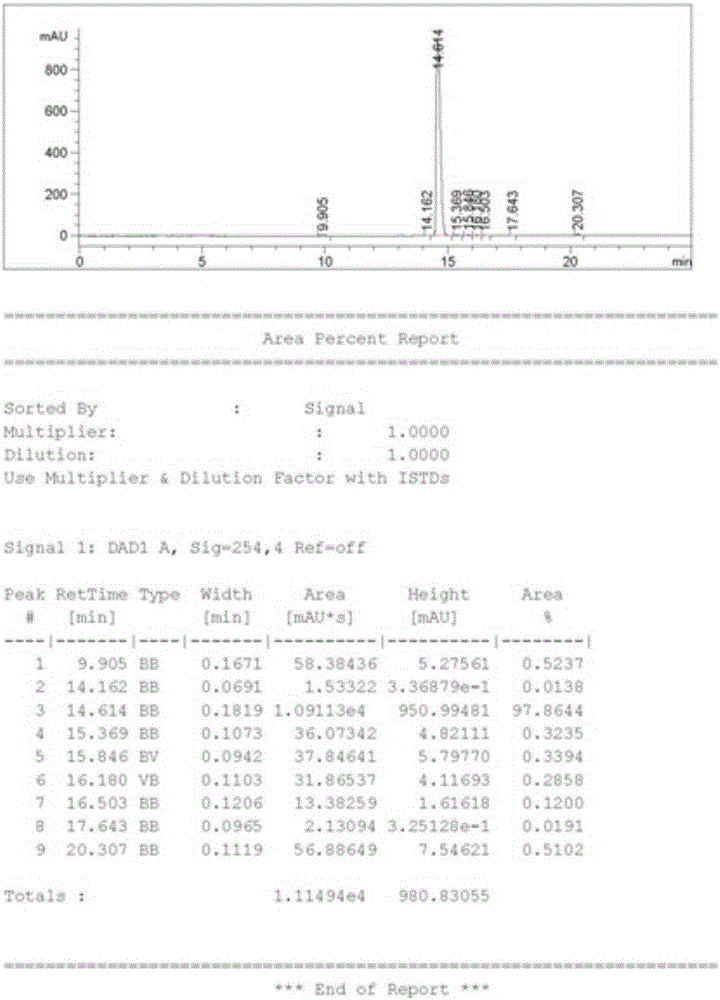
Preparation method of aripiprazole photodegradation impurities
An aripiprazole and photodegradation technology is applied in the field of drug synthesis and achieves the effects of high yield, easy availability of raw materials and simple operation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Add 16.2g of 2,3-dichloroaniline into a 500mL reaction flask, add 160mL of toluene to dissolve, add 15.4g of 1-bromo-2-nitroethane while stirring, then add 0.23g of palladium acetate and 1.2g of BINAP, and reflux reaction 12h. The reaction solution was cooled to room temperature, diluted by adding 80 mL of methyl tert-butyl ether, then suction filtered and concentrated to obtain a crude product, which was then purified by 80 mL of ethyl acetate and 160 mL to obtain 18.8 g of compound IV with a yield of about 80%.
[0040] (2) Take 11.75g of compound IV and put it into a 250mL reaction flask, add 30mL of water and 90mL of ethanol, add 11.2g of iron powder and 5.4g of ammonium chloride under stirring, and reflux for 4h. Then it was filtered while it was hot, solids were precipitated during the cooling down process, and filtered with suction, the resulting filter cake was beaten with 50 mL of ethanol, filtered with suction, and dried to obtain 8.7 g of compound II wi...
Embodiment 2
[0043] (1) Add 16.2g of 2,3-dichloroaniline into a 500mL reaction flask, add 160mL of dichloromethane to dissolve, add 8.9g of nitroacetaldehyde under stirring, then add 30g of sodium triacetylborohydride, reflux for 6 hours, add water Quenches the reaction. After extraction, the organic layer was taken, dried with anhydrous sodium sulfate, filtered, and rotary evaporated to obtain 19.9 g of compound IV with a yield of 85%.
[0044] (2) Add 14.1g of compound IV into a 250mL reaction flask, add 140mL of methanol, add 0.3g of 10% palladium carbon under stirring, under the condition of adding hydrogen, reflux for 8h, heat filter, and rotary steam to obtain 11.1g of compound II , the yield is about 90%.
[0045] (3) Add 6.2g of compound II and 9g of compound III into a 250mL reaction flask, add 120mL of acetonitrile, add 4.15g of potassium carbonate and 7g of sodium iodide under stirring, and reflux for 3h. Then cooled to room temperature, solids were precipitated, filtered with...
Embodiment 3
[0047] (1) Add 16.2g of 2,3-dichloroaniline into a 500mL reaction flask, add 160mL of pyridine to dissolve, add 12.4g of 2-bromoethylamine under stirring, then add 4g of fresh copper oxide and 13.8g of potassium carbonate, and reflux for 12h. The reaction solution was cooled to room temperature, then suction filtered, concentrated to obtain a crude product, and then subjected to column chromatography (eluent: dichloromethane:methanol = 8:1) to obtain 17.6 g of compound II, with a yield of about 75%.
[0048] (2) Add 6.2g of compound II and 9g of compound III into a 250mL reaction flask, add 120mL of acetonitrile, add 12.45g of potassium carbonate and 42g of sodium iodide under stirring, and reflux for 3h. Then cooled to room temperature, solids were precipitated, filtered with suction, the filter cake was added to 50 mL of water and 50 mL of methanol for slurry for 1 h, filtered with suction, and dried to obtain 11.2 g of compound I with a yield of 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com