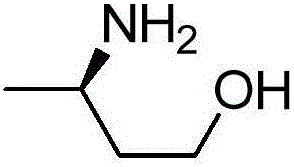
Improved transaminase and application thereof in preparation of (R)-3-aminobutanol
A transaminase and transaminase technology, applied in the application, transferase, enzyme and other directions, can solve the problems of inability to meet the needs of industrial production, increase operation and cost, increase the discharge of industrial three wastes, etc., to achieve good industrial application prospects and reduce production. Cost, product chiral purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Construction of transaminase mutant library
[0033] The sequence of the whole gene synthesis is shown in SEQ ID No.1, and two restriction sites, NdeI and HindIII, were selected and inserted into the pET24a expression vector, and the obtained recombinant expression vector was named pET24a-AtAT. To construct the mutant library, we designed the following 6 primers, see Table 1 for details:
[0034] Table 1 PCR primer table
[0035]
[0036] Use pET24a-AtAT as a template and use the above primers to carry out PCR amplification. The PCR system is: 10×PCR buffer is 5uL, 2.5mM dNTP is 4uL, pfu DNA Polymerse is 0.5uL, pET24a-AtAT template is 0.5uL (including DNA template 0.2 ug), ddH 2O is 36uL, and the AtAT-up upstream primer (SEQ ID No.9) and H55-down downstream primer (SEQ ID No.12), H55-up upstream primer (SEQ ID No.11) and S215- 2uL (10umol / L) of the down downstream primer (SEQ ID No.14), the S215-up upstream primer (SEQ ID No.13) and the AtAT-down downstr...
Embodiment 2
[0037] Example 2 Expression and Screening of Transaminase Mutants
[0038] Inoculate 100uL of bacterial liquid from each well of the overnight-cultivated mutant library into a new 96-well plate, which contains 1mL of fresh LB+kanamycin medium in each well, and culture on a shaker at 37°C until OD 600 When the value reaches 0.8-1.0, add IPTG to a final concentration of 1.0 mM, induce culture at 25°C for about 20 hours, discard the supernatant by centrifugation, and collect the bacteria. Add 400uL reaction solution to each well, which includes: 20g / L substrate 4-hydroxyl-2-butanone, 60g / L isopropylamine, 1mM coenzyme PLP, 100mM potassium phosphate buffer (pH=8.0), at 30 After 24 hours of shaking reaction at ℃, add 10M NaOH to each well to adjust the pH>13, add an equal volume of ethyl acetate for extraction, and after centrifugation, absorb the organic layer for GC analysis to detect the enzyme activity of the mutant. GC conditions: Agilent 7820A gas chromatograph; RESTEK (30...
Embodiment 3
[0039] Example 3 Expression of AtATmut transaminase and preparation of enzyme powder
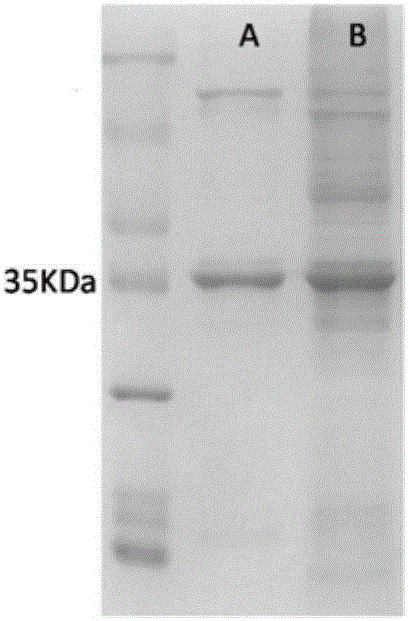
[0040] Transfer the glycerol-preserved strain BL21(DE3) / pET24a-AtATmut to 5 mL of LB test tube medium containing kanamycin for activation culture (cultivate at 37°C for 12 hours), and transfer the activated culture to 400 mL of kanamycin-containing In LB liquid medium of namycin, culture at 37° C. to an OD of 0.6-0.8, add IPTG (final concentration 0.1 mM) and induce culture at 25° C. for 16 hours. Collect the bacteria by centrifugation, resuspend the bacteria in 40mL phosphate buffer (10mM, pH 7.5), and ultrasonically disrupt in an ice-water bath for 15min, and collect the supernatant and precipitate by centrifugation. exists (see figure 2 ), the supernatant was pre-frozen at -20°C, vacuum freeze-dried for 48 hours, and crushed to obtain AtATmut transaminase enzyme powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com