Preparation method of polyvinyl acetal based solid electrolyte
A polyvinyl acetal, solid electrolyte technology, used in circuits, electrical components, secondary batteries, etc., can solve the problem of reduced tensile strength of solid electrolytes, reduced flexibility of polymer segments, and difficult to maintain electrical conductivity. Improvement and other issues, to achieve the effects of excellent macro and micro morphology, improved purity, and increased quantity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
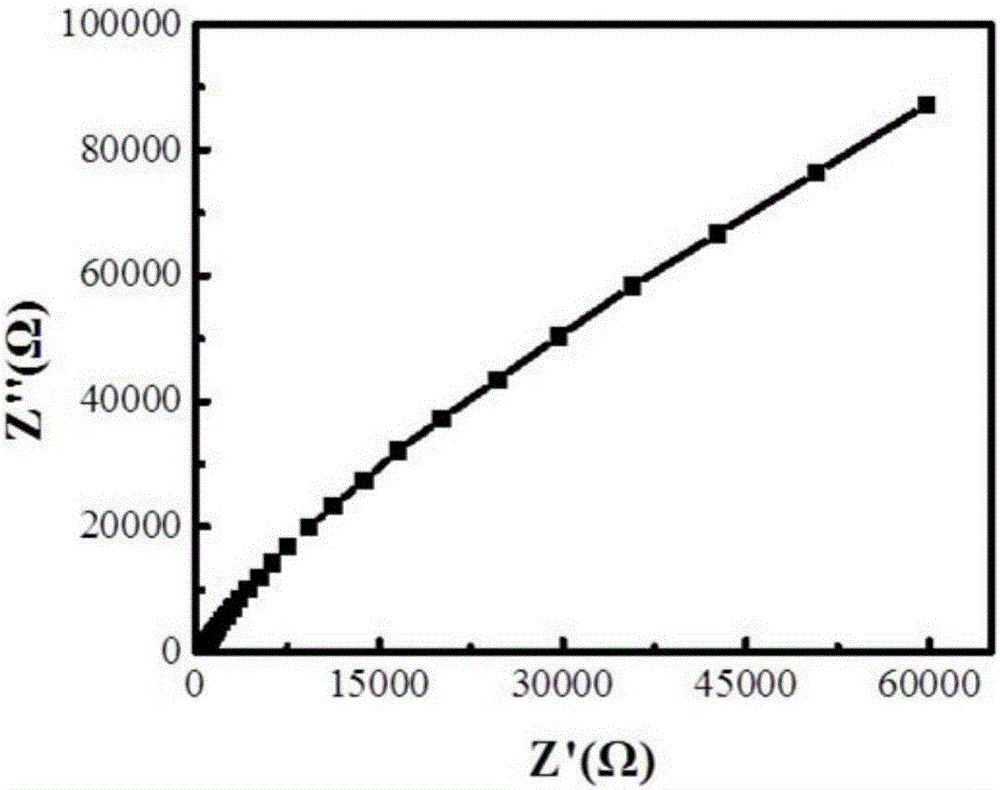
Image
Examples
Embodiment 1
[0028] (1) Take 0.8353g polyvinyl formal (molecular weight is 60000-80000, alcoholysis degree is 79%) and dissolve it in 5.5464g dimethyl sulfoxide, wherein the mass ratio of polyvinyl acetal to organic solvent is 1: 6.64, fully stir to dissolve completely, and prepare a homogeneous solution. Add 0.0284g of boric acid to the above solution (the molar ratio of hydroxyl group to boron atom is 2:1), stir magnetically at 70°C for 5 hours, then add 0.0187g of lithium carbonate and 0.0114g of oxalic acid (boron atom: lithium atom: oxalic acid) The molar ratio is 2:1:2). The water bath was stirred at 90°C for 24 hours, and finally cooled to room temperature to prepare solution system 1;
[0029] (2) Dissolve 0.0500g polyvinyl alcohol (molecular weight is 105000, alcoholysis degree is 98%-99%, pH value is 5-7) in 0.3322g dimethyl sulfoxide, wherein the mass ratio of polyvinyl alcohol to organic solvent The ratio was 1:6.64, and it was fully dissolved by stirring to obtain a homogene...
Embodiment 2
[0041] (1) Dissolve 0.8353g polyvinyl formal (molecular weight 60000-80000, alcoholysis degree 79%) in 8.5353g N,N-dimethylformamide, wherein the mass of polyvinyl acetal and organic solvent The ratio was 1:10, and the mixture was fully dissolved by stirring to obtain a homogeneous solution. Add 0.0321 g of boric acid to the above solution (wherein the molar ratio of hydroxyl and boron atoms is 2:1), stir magnetically at 70°C for 5 hours, then add 0.0122 g of lithium hydroxide and 0.0639 g of oxalic acid (boron atom: lithium atom: The molar ratio of oxalic acid is 2:1:2). The water bath was stirred at 90°C for 24 hours, and finally cooled to room temperature to prepare solution system 1;
[0042] (2) The solution system prepared in step (1) was washed three times with deionized water, suction filtered, and then vacuum-dried at 100 ° C for 14 hours to remove water to obtain a solid product; there is no preparation of solution system 2 here, that is, according to claim 1 In th...
Embodiment 3
[0047] (1) Dissolve 0.8597g of polyvinyl butyral (molecular weight is about 70000) in 6.5939g of N,N-dimethylformamide, wherein the mass ratio of polyvinyl acetal to organic solvent is 1: 7.67, Stir well to dissolve completely, and prepare a homogeneous solution. In the above solution, 0.0124g of boric acid (equivalent to a molar ratio of hydroxyl and boron atoms of 5:1) was stirred magnetically at 60 °C for 5 hours, and then 0.0044g of methyllithium and 0.0180g of oxalic acid (boron atom: lithium atom: oxalic acid) were added. The molar ratio is 2:1:2). The water bath was stirred at 90°C for 24 hours, and finally cooled to room temperature to prepare solution system 1;
[0048] (2) Dissolve 0.1174g polyvinyl alcohol (molecular weight is 110000, alcoholysis degree is 85%-88%, pH value is 6-8)) and dissolve in 1.4088g N,N-dimethylformamide, among which polyvinyl alcohol The mass ratio to the organic solvent is 1:12, and it is fully dissolved by stirring to obtain a homogeneou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com